Ann Surg Treat Res.
2017 Jul;93(1):50-56. 10.4174/astr.2017.93.1.50.
Evaluation of botulinum toxin type A effectiveness in preventing postoperative intraperitoneal adhesions
- Affiliations
-
- 1Department of Emergency Medicine, Necip Fazil City Hospital, Kahramanmaras, Turkey. drdokur@gmail.com
- 2Department of General Surgery, Sanko University School of Medicine, Gaziantep, Turkey.
- KMID: 2392283
- DOI: http://doi.org/10.4174/astr.2017.93.1.50
Abstract
- PURPOSE
Postoperative intraperitoneal adhesions (PIAs) are one of the most important problems surgeons have to face after laparotomies. In this study, we aimed to evaluate the effectiveness of local application of botulinum toxin type A (BoNT-A) in various dosages on the prevention of intra-abdominal adhesions in rats with experimental intra-abdominal adhesions.
METHODS
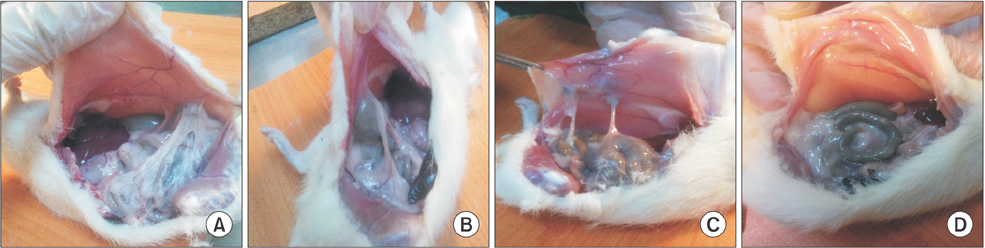
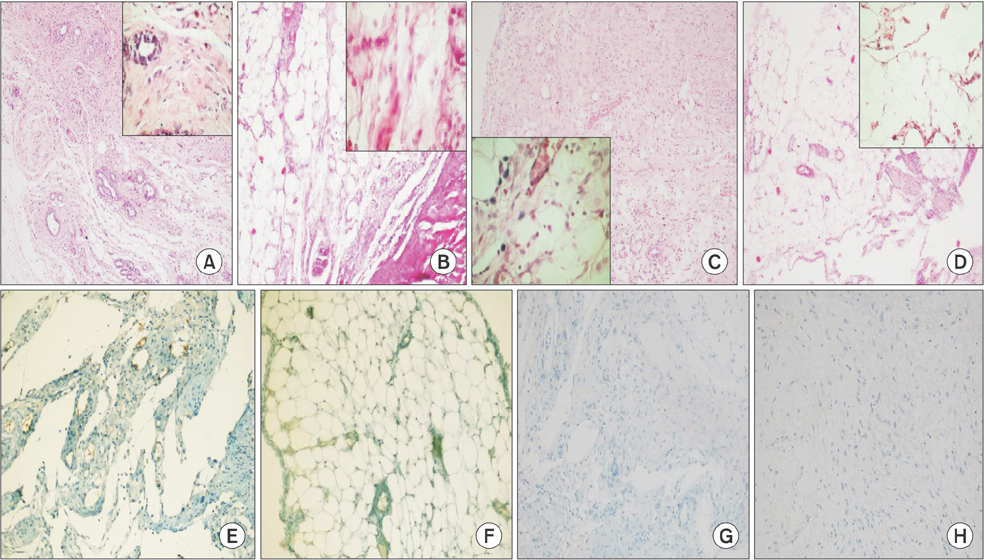
Forty Wistar Albino female rats were randomly separated into 4 groups. The 4 groups were determined as follows: Control (group 1, n = 10); Sham (group 2, n = 10); 10-µg/kg low-dose BoNT-A (group 3, n = 10) and 30-µg/kg high-dose BoNT-A (group 4, n = 10). Subserosal injuries were created on the caecum of all rats. Laparotomy was performed on the fifth day. Adhesion scores, histopathological examination, and E-cadherin expression levels were evaluated.
RESULTS
General adhesion scores for groups 1 and 2 were determined to be significantly high when compared to group 4 (P < 0.001). A significant difference was also determined between groups 3 and 4 in terms of general adhesion scores (P < 0.05). In pair comparisons, a significant decrease in high-dose BoNT-A group (group 4) when compared to groups 1 and 2 in terms of neovascularization, fibroblast density, collagen deposition and inflammatory cell count was determined (P < 0.05).
CONCLUSION
A significant decrease was observed only in postoperative PIAs in the high-dose BoNT-A group between all 4 rat-groups with experimentally created postoperative PIAs. In this study, high-dose BoNT-A is determined to be an effective agent in preventing postoperative PIAs.
Keyword
Figure
Reference
-
1. Ouaissi M, Gaujoux S, Veyrie N, Deneve E, Brigand C, Castel B, et al. Post-operative adhesions after digestive surgery: their incidence and prevention: review of the literature. J Visc Surg. 2012; 149:e104–e114.2. Takagi K, Araki M, Fukuoka H, Takeshita H, Hidaka S, Nanashima A, et al. Novel powdered anti-adhesion material: preventing postoperative intra-abdominal adhesions in a rat model. Int J Med Sci. 2013; 10:467–474.3. Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998; 186:1–9.4. Karaca G, Aydin O, Pehlivanli F, Kocael A, Pekcici R, Duymus E, et al. Effect of ankaferd blood stopper in experimental peritoneal adhesion model. Ann Surg Treat Res. 2016; 90:213–217.5. Kirdak T, Uysal E, Korun N. Assessment of effectiveness of different doses of methylprednisolone on intraabdominal adhesion prevention. Ulus Travma Acil Cerrahi Derg. 2008; 14:188–191.6. Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: the adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004; 11:307–314.7. Jin X, Ren S, Macarak E, Rosenbloom J. Pathobiological mechanisms of peritoneal adhesions: The mesenchymal transition of rat peritoneal mesothelial cells induced by TGF-β1 and IL-6 requires activation of Erk1/2 and Smad2 linker region phosphorylation. Matrix Biol. 2016; 51:55–64.8. Kim YJ, Kim JH, Lee KJ, Choi MM, Kim YH, Rhie GE, et al. Botulinum neurotoxin type A induces TLR2-mediated inflammatory responses in macrophages. PLoS One. 2015; 10:e0120840.9. Linsky CB, Diamond MP, Cunningham T, Constantine B, DeCherney AH, diZerega GS. Adhesion reduction in the rabbit uterine horn model using an absorbable barrier, TC-7. J Reprod Med. 1987; 32:17–20.10. Ehrlich HP, Tarver H, Hunt TK. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann Surg. 1973; 177:222–227.11. Hsu YC, Wang HJ, Chuang YC. Intraprostatic Botulinum Neurotoxin Type A Injection for Benign Prostatic Hyperplasia-A Spotlight in Reality. Toxins (Basel). 2016; 8:pii: E126.12. Nelson RL. Efficacy of Fissurectomy and Botox for Chronic Anal Fissure. Dis Colon Rectum. 2016; 59:e41.13. Houston FE, Hain BA, Adams TJ, Houston KL, O'Keeffe R, Dodd SL. Heat shock protein 70 overexpression does not attenuate atrophy in botulinum neurotoxin type A-treated skeletal muscle. J Appl Physiol (1985). 2015; 119:83–92.14. Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology. 2007; 70:463–468.15. O'Neil LM, Palme CE, Riffat F, Mahant N. Botulinum Toxin for the Management of Sjogren Syndrome-Associated Recurrent Parotitis. J Oral Maxillofac Surg. 2016; 74:2428–2430.16. Lackovic Z, Filipovic B, Matak I, Helyes Z. Activity of botulinum toxin type A in cranial dura: implications for treatment of migraine and other headaches. Br J Pharmacol. 2016; 173:279–291.17. Aoishi K, Takahashi H, Hato N, Gyo K, Yokota M, Ozaki S, et al. Treatment of allergic rhinitis with intranasal infusion of botulinum toxin type A in mice. Life Sci. 2016; 147:132–136.18. Kim SY, Lee SH, Lee B, Park YJ, Park JH, Lee YS, et al. The protective effects of botulinum Toxin A against f lap necrosis after perforator twisting and its underlying molecular mechanism in a rat model. Ann Plast Surg. 2016; 77:242–248.19. Lee BJ, Jeong JH, Wang SG, Lee JC, Goh EK, Kim HW. Effect of botulinum toxin type a on a rat surgical wound model. Clin Exp Otorhinolaryngol. 2009; 2:20–27.20. Molinas CR, Binda MM, Manavella GD, Koninckx PR. Adhesion formation after laparoscopic surgery: what do we know about the role of the peritoneal environment. Facts Views Vis Obgyn. 2010; 2:149–160.21. Boys F. The prophylaxis of peritoneal adhesion. Review of literature. Surgery. 1942; 11:118–168.22. Xiao Z, Zhang F, Lin W, Zhang M, Liu Y. Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: a preliminary report. Aesthetic Plast Surg. 2010; 34:424–427.23. Oh SH, Lee Y, Seo YJ, Lee JH, Yang JD, Chung HY, et al. The potential effect of botulinum toxin type A on human dermal fibroblasts: an in vitro study. Dermatol Surg. 2012; 38:1689–1694.24. Kim S, Ahn M, Piao Y, Ha Y, Choi DK, Yi MH, et al. Effect of botulinum toxin type A on TGF-β/Smad pathway signaling: implications for silicone-induced capsule-formation. Plast Reconstr Surg. 2016; 138:821e–829e.25. Kim YS, Hong JW, Yoon JH, Hwang YS, Roh TS, Rah DK. Botulinum toxin A affects early capsule formation around silicone implants in a rat model. Ann Plast Surg. 2015; 74:488–495.26. Sahinkanat T, Ozkan KU, Ciralik H, Ozturk S, Resim S. Botulinum toxin-A to improve urethral wound healing: an experimental study in a rat model. Urology. 2009; 73:405–409.27. Minamoto VB, Suzuki KP, Bremner SN, Lieber RL, Ward SR. Dramatic changes in muscle contractile and structural properties after 2 botulinum toxin injections. Muscle Nerve. 2015; 52:649–657.28. Gauglitz GG, Bureik D, Dombrowski Y, Pavicic T, Ruzicka T, Schauber J. Botulinum toxin A for the treatment of keloids. Skin Pharmacol Physiol. 2012; 25:313–318.29. Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001; 7:556–566.30. Lee K, Zhong X, Gu S, Kruel AM, Dorner MB, Perry K, et al. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science. 2014; 344:1405–1410.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A clinical study on the use of botulinum toxin type a in maxillofacial area
- Botulinum Toxin-Type A in Cervical Myofascial Pain Syndrome: A report of 3 cases
- Treatment of Winkles and Hyperhidrosis with Botulinum Toxin Type A
- Effectiveness of Botulinum Toxin A in Treatment of Frey's Syndrome
- The Effect of Botulinum Toxin A Injection in the Upper Eyelid Retraction