Ann Rehabil Med.
2017 Jun;41(3):475-482. 10.5535/arm.2017.41.3.475.
Research Designs and Statistical Methods Trends in the Annals of Rehabilitation Medicine
- Affiliations
-
- 1Department of Rehabilitation Medicine, Konkuk University School of Medicine, Chungju, Korea. kimnerve@hanmail.net
- 2Department of Radiology, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2389464
- DOI: http://doi.org/10.5535/arm.2017.41.3.475
Abstract
OBJECTIVE
To investigate trends of the research designs and statistical methods in the Annals of Rehabilitation Medicine (ARM) published from 2005 to 2015 through a comparison of articles with the Archives of Physical Medicine and Rehabilitation (APMR).
METHODS
The authors reviewed all articles published in ARM and APMR for the years 2005 and 2015 in order to determine their research designs as well as their statistical methods used in each article.
RESULTS
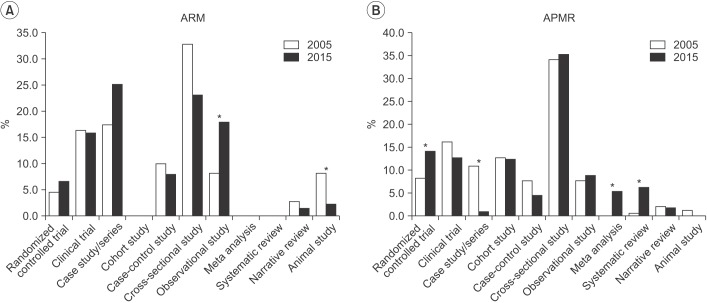
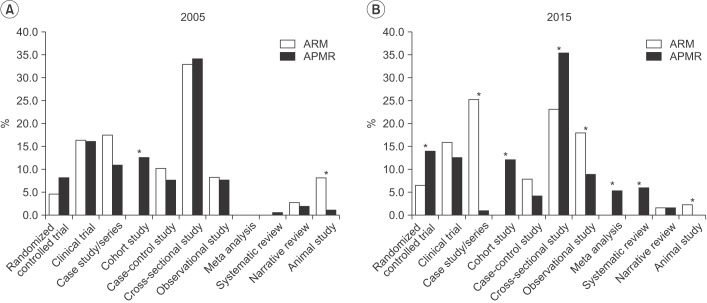
In ARM, randomized controlled trials increased from 4.5% in 2005 to 6.5% in 2015. In APMR, randomized controlled trials increased from 8.1% in 2005 to 14.0% in 2015, meta-analyses increased to 5.3%, and systematic reviews increased to 6%. The number of studies using statistical methods increased in ARM from 1.9 to 2.6 per article and in APMR, from 2.7 to 3.1. Use of advanced methods in ARM also showed an increase from 2005 to 2015.
CONCLUSION
This study concludes that there is a trend of increased awareness and attempts to use varied research approaches in ARM articles. There should also be more in-depth discussions and opportunities for researchers to share their experiences regarding statistical methods in the clinical field.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Bibliometric Analysis Using Alternative Metrics for Articles in the
Annals of Rehabilitation Medicine
Seok Cheol Han, Hyo Jung Kang, Won Jae Lee, Hee Sup Chung, Jong Hyuk Lee
Ann Rehabil Med. 2020;44(2):158-164. doi: 10.5535/arm.2020.44.2.158.
Reference
-
1. Mellis C. Evidence-based medicine: what has happened in the past 50 years? J Paediatr Child Health. 2015; 51:65–68. PMID: 25536873.
Article2. Swennen MH, van der Heijden GJ, Boeije HR, van Rheenen N, Verheul FJ, van der Graaf Y, et al. Doctors' perceptions and use of evidence-based medicine: a systematic review and thematic synthesis of qualitative studies. Acad Med. 2013; 88:1384–1396. PMID: 23887011.3. Shaughnessy AF, Torro JR, Frame KA, Bakshi M. Evidence-based medicine teaching requirements in the USA: taxonomy and themes. J Evid Based Med. 2016; 9:53–58. PMID: 27310370.
Article4. Blanco MA, Capello CF, Dorsch JL, Perry G, Zanetti ML. A survey study of evidence-based medicine training in US and Canadian medical schools. J Med Libr Assoc. 2014; 102:160–168. PMID: 25031556.
Article5. Ma X, Xu B, Liu Q, Zhang Y, Xiong H, Li Y. Effectiveness of evidence-based medicine training for undergraduate students at a Chinese Military Medical University: a self-controlled trial. BMC Med Educ. 2014; 14:133. PMID: 24996537.
Article6. Sanchez-Mendiola M, Kieffer-Escobar LF, Marin-Beltran S, Downing SM, Schwartz A. Teaching of evidence-based medicine to medical students in Mexico: a randomized controlled trial. BMC Med Educ. 2012; 12:107. PMID: 23131115.
Article7. Meats E, Heneghan C, Crilly M, Glasziou P. Evidence-based medicine teaching in UK medical schools. Med Teach. 2009; 31:332–337. PMID: 19404893.
Article8. Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992; 268:2420–2425. PMID: 1404801.9. Kim JH, Kim JS. Statistical methods used in the Journal of Korean Academy of Rehabilitation Medicine. J Korean Acad Rehabil Med. 1998; 22:46–55.10. Fukui T, Rahman M, Sekimoto M, Hira K, Maeda K, Morimoto T, et al. Study design, statistical method, and level of evidence in Japanese and American clinical journals. J Epidemiol. 2002; 12:266–270. PMID: 12164331.
Article11. Afshar K, Jafari S, Seth A, Lee JK, MacNeily AE. Publications by the American Academy of Pediatrics Section on Urology: the quality of research design and statistical methodology. J Urol. 2009; 182(4 Suppl):1906–1910. PMID: 19695587.
Article12. Huang W, LaBerge JM, Lu Y, Glidden DV. Research publications in vascular and interventional radiology: research topics, study designs, and statistical methods. J Vasc Interv Radiol. 2002; 13:247–255. PMID: 11875084.
Article13. Baumberger JP, Bangert AW. Research designs and statistical techniques used in the Journal of Learning Disabilities, 1989-1993. J Learn Disabil. 1996; 29:313–316. PMID: 8732893.
Article14. Hassan S, Yellur R, Subramani P, Adiga P, Gokhale M, Iyer MS, et al. Research design and statistical methods in Indian medical journals: a retrospective survey. PLoS One. 2015; 10:e0121268. PMID: 25856194.
Article15. Akhtar S, Shah SW, Rafiq M, Khan A. Research design and statistical methods in Pakistan Journal of Medical Sciences (PJMS). Pak J Med Sci. 2016; 32:151–154. PMID: 27022365.
Article16. Jin Z, Yu D, Zhang L, Meng H, Lu J, Gao Q, et al. A retrospective survey of research design and statistical analyses in selected Chinese medical journals in 1998 and 2008. PLoS One. 2010; 5:e10822. PMID: 20520824.
Article17. Wang Q, Zhang B. Research design and statistical methods in Chinese medical journals. JAMA. 1998; 280:283–285. PMID: 9676683.
Article18. US Preventive Services Task Force. Procedure manual [Internet]. Rockville: US Preventive Services Task Force;2015. cited 2017 May 1. Available from: http://www.uspreventiveservicestaskforce.org/Page/Name/methods-and-processes.19. Oxford Centre for Evidence-Based Medicine - levels of evidence [Internet]. Oxford: Centre for Evidence-Based Medicine;2009. cited 2017 May 1. Available from: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009.20. Rennie D. Improving reports of studies of diagnostic tests: the STARD initiative. JAMA. 2003; 289:89–90. PMID: 12503983.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Trends in Rehabilitation for Cancer Patients
- Changes in Epidemiological Trends and Rehabilitation Usage in Neurological Diseases in Korea: Stroke
- Statistical Methods Used in the Journal of Korean Academy of Rehabilitation Medicine
- A Review of Community Health Nursing Research in Korea and Japan
- Pervasive Misclassification and Misconception of Study Designs in Asian Dermatology Journals Listed in Science Citation Index-Expanded