Ann Clin Microbiol.
2017 Jun;20(2):42-51. 10.5145/ACM.2017.20.2.42.
Diagnostic Accuracy and Detection Rate of Real-Time PCR for Detection of Group B Streptococcal Colonization in Pregnant Women: Systemic Review of Literature and Meta-Analysis
- Affiliations
-
- 1Division of New Health Technology Assessment, National Evidence-Based Healthcare Collaborating Agency, Seoul, Korea.
- 2Department of Laboratory Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea. hlseo@cha.ac.kr
- KMID: 2383230
- DOI: http://doi.org/10.5145/ACM.2017.20.2.42
Abstract
- BACKGROUND
Group B streptococcus (Streptococcus agalactiae, GBS) was reported as a major cause of neonatal infection and death. To prevent vertical transmission, CDC recommended that all women in week 35-37 of pregnancy should receive the GBS colonization test. We conducted a systematic review and meta-analysis to evaluate diagnostic accuracy and detection rate of real-time PCR for GBS in pregnant women.
METHODS
The literature review for GBS using real-time PCR was done including KoreaMed, Ovid-MEDLINE, Ovid-EMBASE, and Cochrane Library on November 3, 2015. 443 articles were collected. Two authors select articles and evaluated the quality of studies using Scottish Intercollegiate Guidelines Network tool independently.
RESULTS
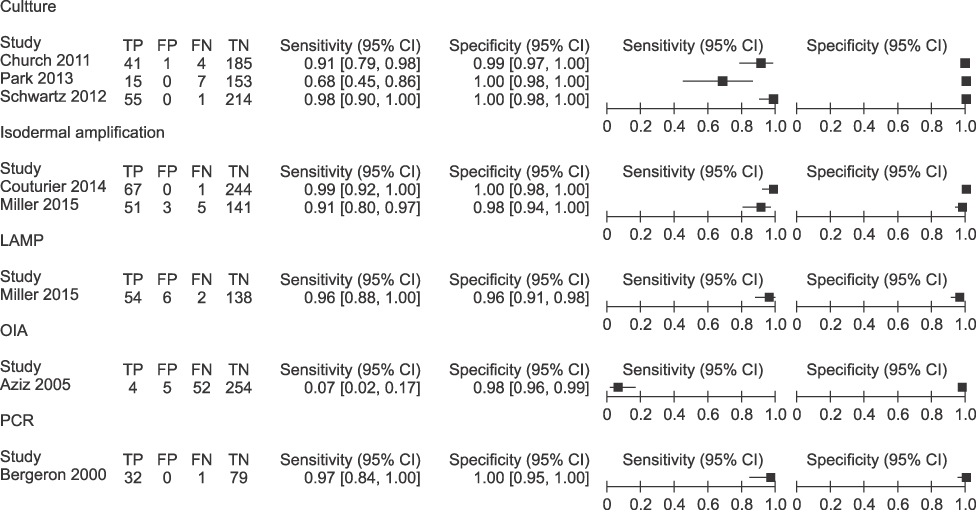
Diagnostic accuracy of the real-time PCR was assessed by meta-analysis through 34 articles (13,516 for real-time PCR, 1,815 for culture and other comparison test). The GBS colonization was assessed through 34 articles, which reported varying values of 2.0-69.2% using real-time PCR. The real-time PCR for GBS was shown to have overall sensitivity of 0.93 (95% CI 0.92-0.94, I2=86.3%), overall specificity of 0.96 (95% CI 0.96-0.96, I2=90.2%), SROC AUC of 0.99.
CONCLUSION
Real-time PCR is an effective test for detecting GBS colonization in pregnant women, resulted in preventing the infection in a new born baby.
Keyword
MeSH Terms
Figure
Reference
-
1. Atkins KL, Atkinson RM, Shanks A, Parvin CA, Dunne WM, Gross G. Evaluation of polymerase chain reaction for group B streptococcus detection using an improved culture method. Obstet Gynecol. 2006; 108:488–491.2. Honest H, Sharma S, Khan KS. Rapid tests for group B Streptococcus colonization in laboring women: a systematic review. Pediatrics. 2006; 117:1055–1066.3. Bergeron MG, Ke D, Ménard C, Picard FJ, Gagnon M, Bernier M, et al. Rapid detection of group B streptococci in pregnant women at delivery. N Engl J Med. 2000; 343:175–179.4. Choi CW. Neonatal group B streptococcal diseases. Korean J Perinatol. 2012; 23:133–142.5. Oh CE. Group B streptococcal disease in Korean neonates. Korean J Pediatr Infect Dis. 2012; 19:43–54.6. Park JS, Cho DH, Yang JH, Kim MY, Shin SM, Kim EC, et al. Usefulness of a rapid real-time PCR assay in prenatal screening for group B streptococcus colonization. Ann Lab Med. 2013; 33:39–44.7. Lee SH, Park KU, Lee HK, Kim MY, Kim JY, Kwon WK, et al. Perineal colonization rate and antimicrobial susceptibility of group B streptococcus in pregnant and non-pregnant Korean women. Korean J Clin Microbiol. 2009; 12:180–185.8. Kim EJ, Oh KY, Kim MY, Seo YS, Shin JH, Song YR, et al. Risk factors for group B streptococcus colonization among pregnant women in Korea. Epidemiol Health. 2011; 33:e2011010.9. Choi SJ, Park SD, Jang IH, Uh Y, Lee A. The prevalence of vaginal microorganisms in pregnant women with preterm labor and preterm birth. Ann Lab Med. 2012; 32:194–200.10. Aziz N, Baron EJ, D'Souza H, Nourbakhsh M, Druzin ML, Benitz WE. Comparison of rapid intrapartum screening methods for group B streptococcal vaginal colonization. J Matern Fetal Neonatal Med. 2005; 18:225–229.11. Couturier BA, Weight T, Elmer H, Schlaberg R. Antepartum screening for group B Streptococcus by three FDA-cleared molecular tests and effect of shortened enrichment culture on molecular detection rates. J Clin Microbiol. 2014; 52:3429–3432.12. Verani JR, McGee L, Schrag SJ. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010; 59:1–36.13. Scottish intercollegiate guidelines network. Methodology checklist. last visited on 12 June 2017. http://www.sign.ac.uk/checklists-and-notes.html [Online].14. Scottish intercollegiate guidelines network. SIGN 50: a guideline developer's handbook. last visited on 12 June 2017. http://www.sign.ac.uk/sign-50.html [Online].15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.16. Uh Y, Jang IH, Kwon JY, Kim NS, Kim MC, Park DW, et al. Studies on the usefulness of new Granada selective medium for the detection of group B streptococci in pregnant women. J Wonju Med Coll. 1996; 9:7–15.17. Park LS, Seo K, Kim SK, Park YW, Jung HY, Chong YS, et al. A study of group B streptococcal infection in Korean pregnant women. Korean J Obstet Gynecol. 1999; 42:2038–2042.18. Choi KU, Koh SK, Lee JY, Park JH, Hwang SO, Lee BI, et al. Clinical significance of group B streptococcal infection in pregnant women. Korean J Obstet Gynecol. 2002; 45:811–815.19. Kim MW, Jang HO, Chang DY, Cho JR, Kim YA, Choi HM, et al. Group B streptococcal colonization rate in Korean pregnant women. Korean J Obstet Gynecol. 2006; 49:337–344.20. Buchan BW, Faron ML, Fuller D, Davis TE, Mayne D, Ledeboer NA. Multicenter clinical evaluation of the Xpert GBS LB assay for detection of group B Streptococcus in prenatal screening specimens. J Clin Microbiol. 2015; 53:443–448.21. Miller SA, Deak E, Humphries R. Comparison of the AmpliVue, BD Max System, and illumigene Molecular Assays for Detection of Group B Streptococcus in antenatal screening specimens. J Clin Microbiol. 2015; 53:1938–1941.22. Morozumi M, Chiba N, Igarashi Y, Mitsuhashi N, Wajima T, Iwata S, et al. Direct identification of Streptococcus agalactiae and capsular type by real-time PCR in vaginal swabs from pregnant women. J Infect Chemother. 2015; 21:34–38.23. Yeung SW, Cheung PT, Chau SL, Ip M, Lao TT, Leung TY, et al. Evaluation of an in-house real-time polymerase chain reaction method to identify group B streptococcus colonization in pregnancy. J Obstet Gynaecol Res. 2015; 41:1357–1362.24. Mueller M, Henle A, Droz S, Kind AB, Rohner S, Baumann M, et al. Intrapartum detection of Group B streptococci colonization by rapid PCR-test on labor ward. Eur J Obstet Gynecol Reprod Biol. 2014; 176:137–141.25. Berg BR, Houseman JL, Garrasi MA, Young CL, Newton DW. Culture-based method with performance comparable to that of PCR-based methods for detection of group B Streptococcus in screening samples from pregnant women. J Clin Microbiol. 2013; 51:1253–1255.26. Poncelet-Jasserand E, Forges F, Varlet MN, Chauleur C, Seffert P, Siani C, et al. Reduction of the use of antimicrobial drugs following the rapid detection of Streptococcus agalactiae in the vagina at delivery by real-time PCR assay. BJOG. 2013; 120:1098–1108.27. Feuerschuette OM, Serratine AC, Bazzo ML, Martins TR, Silveira SK, da Silva RM. Performance of RT-PCR in the detection of Streptococcus agalactiae in the anogenital tract of pregnant women. Arch Gynecol Obstet. 2012; 286:1437–1442.28. Schwartz J, Robinson-Dunn B, Makin J, Boyanton BL Jr. Evaluation of the BD MAX GBS assay to detect Streptococcus group B in LIM broth-enriched antepartum vaginal-rectal specimens. Diagn Microbiol Infect Dis. 2012; 73:97–98.29. Church DL, Baxter H, Lloyd T, Miller B, Gregson DB. Evaluation of the Xpert® group B streptococcus real-time polymerase chain reaction assay compared to StrepB Carrot Broth™ for the rapid intrapartum detection of group B streptococcus colonization. Diagn Microbiol Infect Dis. 2011; 69:460–462.30. Young BC, Dodge LE, Gupta M, Rhee JS, Hacker MR. Evaluation of a rapid, real-time intrapartum group B streptococcus assay. Am J Obstet Gynecol. 2011; 205:372.e1–372.e6.31. Alfa MJ, Sepehri S, De Gagne P, Helawa M, Sandhu G, Harding GK. Real-time PCR assay provides reliable assessment of intrapartum carriage of group B Streptococcus. J Clin Microbiol. 2010; 48:3095–3099.32. Jordan JA, Hall G, Davis T. Multicenter study evaluating performance of the Smart Group B Streptococcus (GBS) assay using an enrichment protocol for detecting GBS colonization in patients in the antepartum period. J Clin Microbiol. 2010; 48:3193–3197.33. Riedlinger J, Beqaj SH, Milish MA, Young S, Smith R, Dodd M, et al. Multicenter evaluation of the BD Max GBS assay for detection of group B streptococci in prenatal vaginal and rectal screening swab specimens from pregnant women. J Clin Microbiol. 2010; 48:4239–4241.34. El Helali N, Nguyen JC, Ly A, Giovangrandi Y, Trinquart L. Diagnostic accuracy of a rapid real-time polymerase chain reaction assay for universal intrapartum group B streptococcus screening. Clin Infect Dis. 2009; 49:417–423.35. Scicchitano LM, Bourbeau PP. Comparative evaluation of the AccuProbe Group B Streptococcus Culture Test, the BD GeneOhm Strep B assay, and culture for detection of group B streptococci in pregnant women. J Clin Microbiol. 2009; 47:3021–3023.36. Wei CF, She BC, Liang HS, Ling QD, Tsai CY, Yen CW, et al. Prenatal group B Streptococcus test using real-time polymerase chain reaction. Taiwan J Obstet Gynecol. 2009; 48:116–119.37. Wernecke M, Mullen C, Sharma V, Morrison J, Barry T, Maher M, et al. Evaluation of a novel real-time PCR test based on the ssrA gene for the identification of group B streptococci in vaginal swabs. BMC Infect Dis. 2009; 9:148.38. Block T, Munson E, Culver A, Vaughan K, Hryciuk JE. Comparison of carrot broth- and selective Todd-Hewitt broth-enhanced PCR protocols for real-time detection of Streptococcus agalactiae in prenatal vaginal/anorectal specimens. J Clin Microbiol. 2008; 46:3615–3620.39. Edwards RK, Novak-Weekley SM, Koty PP, Davis T, Leeds LJ, Jordan JA. Rapid group B streptococci screening using a real-time polymerase chain reaction assay. Obstet Gynecol. 2008; 111:1335–1341.40. Money D, Dobson S, Cole L, Karacabeyli E, Blondel-Hill E, Milner R, et al. An evaluation of a rapid real time polymerase chain reaction assay for detection of group B streptococcus as part of a neonatal group B streptococcus prevention strategy. J Obstet Gynaecol Can. 2008; 30:770–775.41. Smith D, Perry JD, Laine L, Galloway A, Gould FK. Comparison of BD GeneOhm real-time polymerase chain reaction with chromogenic and conventional culture methods for detection of group B Streptococcus in clinical samples. Diagn Microbiol Infect Dis. 2008; 61:369–372.42. Bergseng H, Bevanger L, Rygg M, Bergh K. Real-time PCR targeting the sip gene for detection of group B streptococcus colonization in pregnant women at delivery. J Med Microbiol. 2007; 56:223–228.43. Gavino M, Wang E. A comparison of a new rapid real-time polymerase chain reaction system to traditional culture in determining group B streptococcus colonization. Am J Obstet Gynecol. 2007; 197:388.e1–388.e4.44. Goodrich JS, Miller MB. Comparison of culture and 2 real-time polymerase chain reaction assays to detect group B Streptococcus during antepartum screening. Diagn Microbiol Infect Dis. 2007; 59:17–22.45. Chan KL, Levi K, Towner KJ, Weston VC, Ramsay MM, Kean LH. Evaluation of the sensitivity of a rapid polymerase chain reaction for detection of group B streptococcus. J Obstet Gynaecol. 2006; 26:402–406.46. Réglier-Poupet H, Quesne G, Le Théo E, Dommergues M, Berche P, Trieu-Cuot P, et al. Prospective evaluation of a real-time PCR assay for detection of group B streptococci in vaginal swabs from pregnant women. Eur J Clin Microbiol Infect Dis. 2005; 24:355–357.47. Uhl JR, Vetter EA, Boldt KL, Johnston BW, Ramin KD, Adams MJ, et al. Use of the Roche LightCycler Strep B assay for detection of group B streptococcus from vaginal and rectal swabs. J Clin Microbiol. 2005; 43:4046–4051.48. Davies HD, Miller MA, Faro S, Gregson D, Kehl SC, Jordan JA. Multicenter study of a rapid molecular-based assay for the diagnosis of group B Streptococcus colonization in pregnant women. Clin Infect Dis. 2004; 39:1129–1135.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Colonization Rate, Serotypes, and Distributions of Macrolide-Lincosamide-Streptogramin(B) Resistant Types of Group B Streptococci in Pregnant Women
- Clinical Significance of Group B Streptococcal Infection in Pregnant Women
- Usefulness of a Rapid Real-time PCR Assay in Prenatal Screening for Group B Streptococcus Colonization
- A Study of Group B Streptococcal Infection in Korean Pregnant Women
- Diagnostic Effectiveness of PCR-based Tests Detecting BRAF Mutation for Treating Malignant Melanoma: A Systematic Review