J Pathol Transl Med.
2015 May;49(3):249-256. 10.4132/jptm.2015.03.27.
MUC2 Expression Is Correlated with Tumor Differentiation and Inhibits Tumor Invasion in Gastric Carcinomas: A Systematic Review and Meta-analysis
- Affiliations
-
- 1Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. jhpath.sohn@samsung.com
- 2Department of Pathology, Inje University Sanggye Paik Hospital, Seoul, Korea.
- KMID: 2381384
- DOI: http://doi.org/10.4132/jptm.2015.03.27
Abstract
- BACKGROUND
While MUC2 is expressed in intestinal metaplasia and malignant lesions, the clinicopathological significance of MUC2 expression is not fully elucidated in gastric carcinoma (GC).
METHODS
The present study investigated the correlation between MUC2 expression and clinicopathological parameters in 167 human GCs. In addition, to confirm the clinicopathological significance of MUC2 expression, we performed a systematic review and meta-analysis in 1,832 GCs.
RESULTS
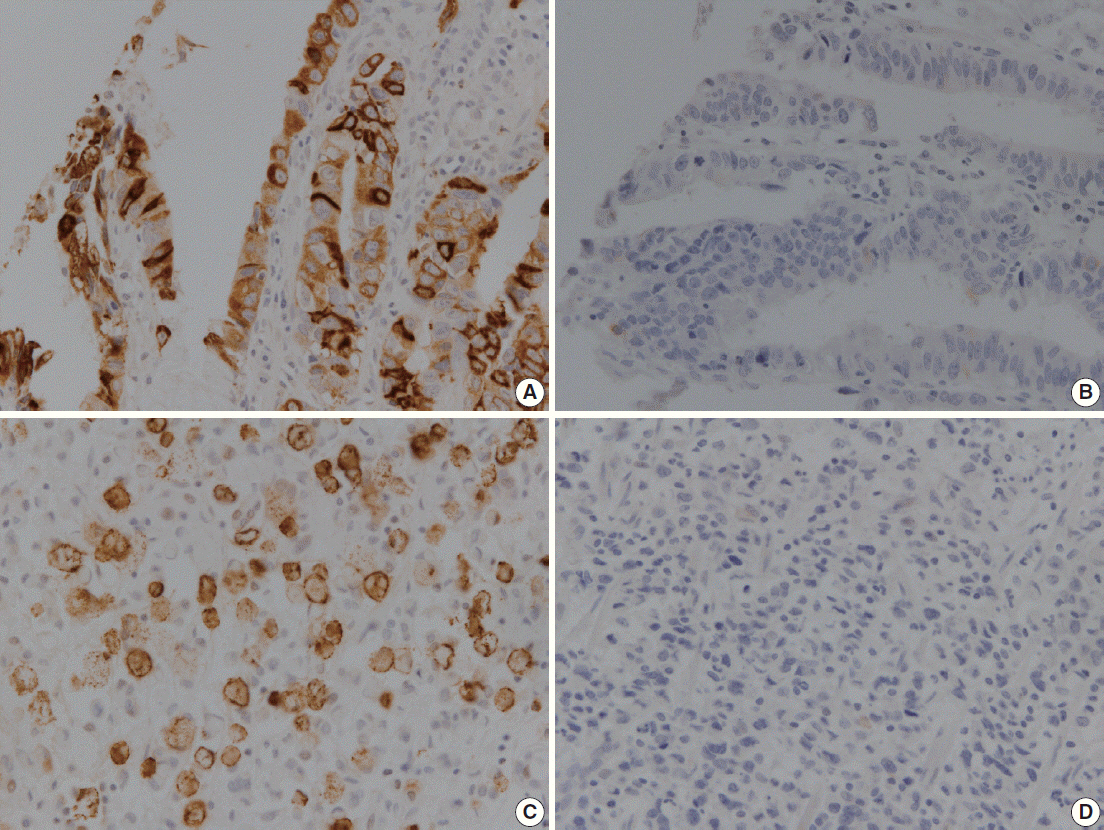
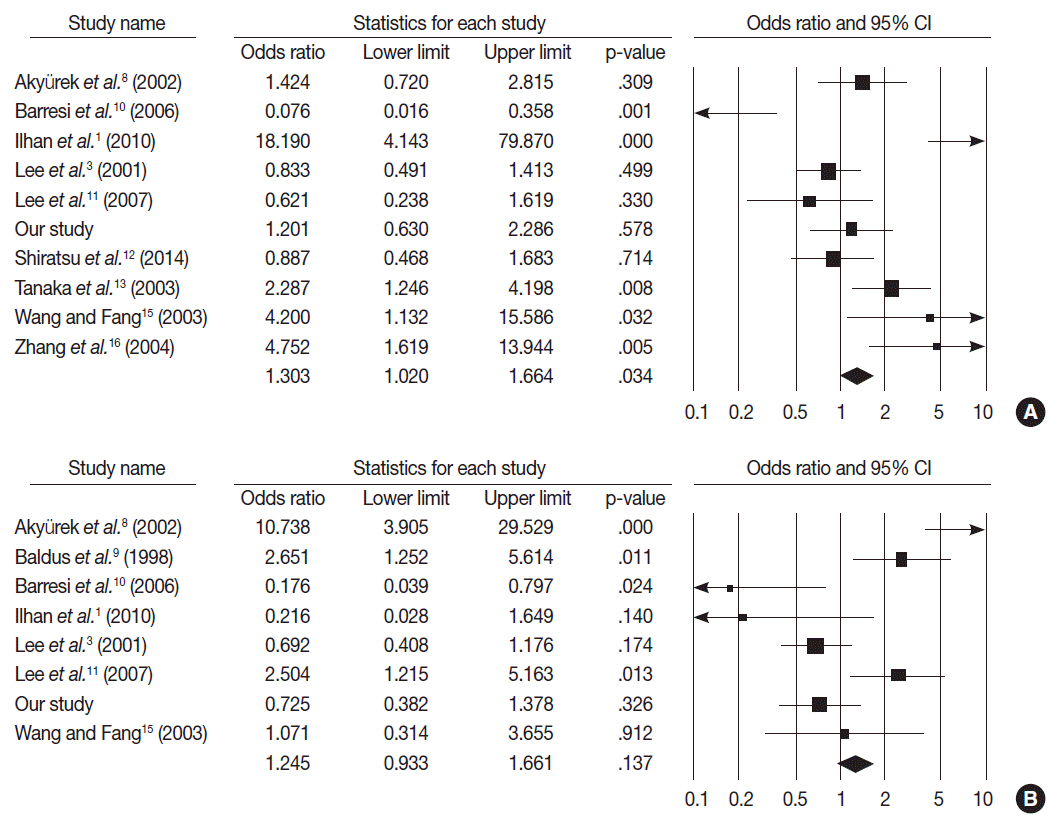
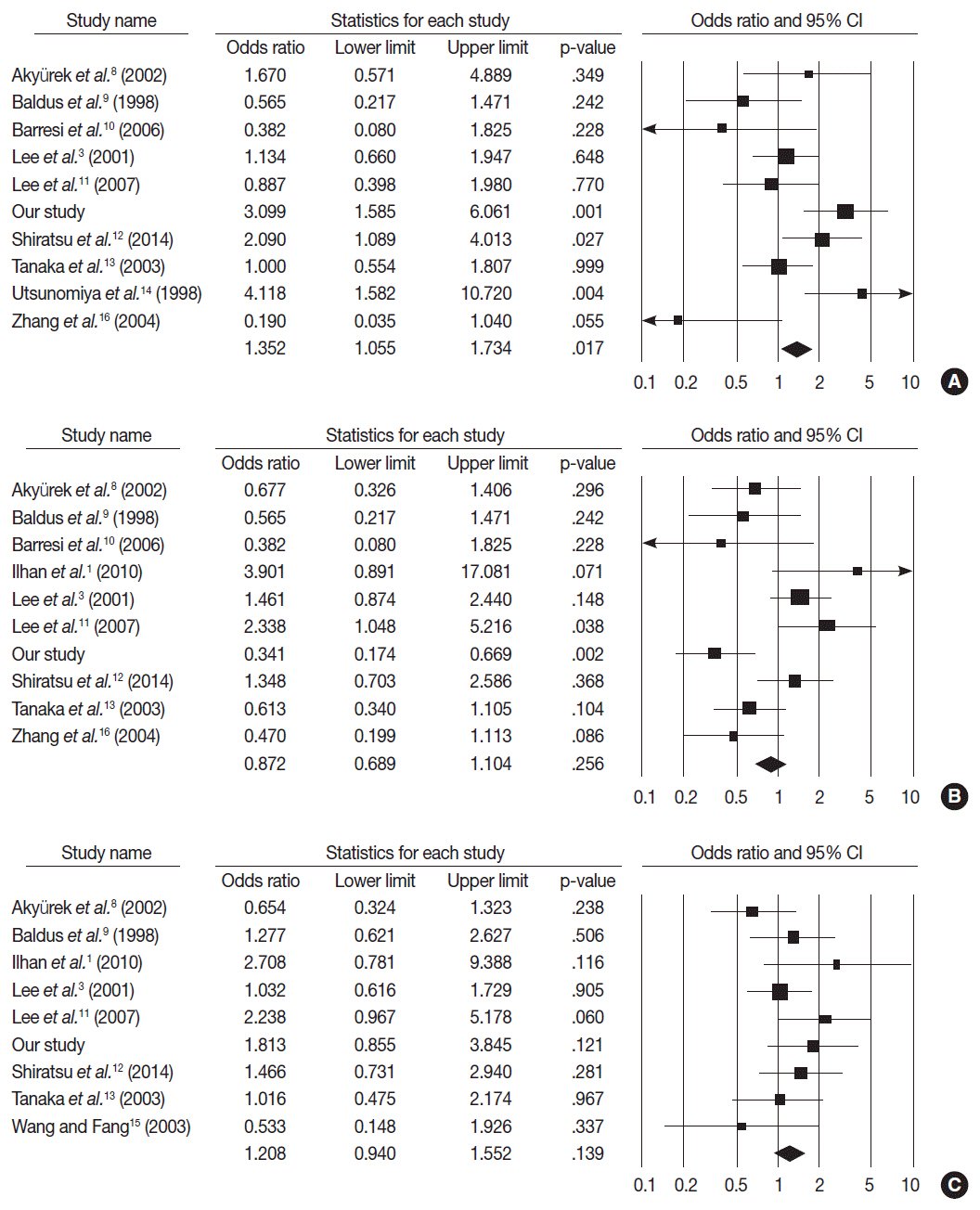
MUC2 expression was found in 58 of 167 GCs (34.7%). MUC2-expressing GC showed lower primary tumor (T), regional lymph node (N), and tumor node metastasis (TNM) stages compared with GCs without MUC2 expression (p=.001, p=.001, and p=.011, respectively). However, MUC2 expression was not correlated with Lauren's classification and tumor differentiation. In meta-analysis, MUC2 expression was significantly correlated with differentiation and lower tumor stage (odds ratio [OR], 1.303; 95% confidence interval [CI], 1.020 to 1.664; p = .034 and OR, 1.352; 95% CI, 1.055 to 1.734; p = .017, respectively) but not with Lauren's classification, pN stage, or pTNM stage.
CONCLUSIONS
MUC2 expression was correlated with a lower tumor depth and lower lymph node metastasis in our study; the meta-analysis showed a correlation of MUC2 expression with tumor differentiation and lower tumor depth.
Figure
Reference
-
1. Ïlhan Ö, Han Ü, Önal B, Çelik SY. Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk J Gastroenterol. 2010; 21:345–52.
Article2. Kim JY, Shin NR, Kim A, et al. Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol. 2013; 47:28–35.
Article3. Lee HS, Lee HK, Kim HS, Yang HK, Kim YI, Kim WH. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer. 2001; 92:1427–34.4. Wakatsuki K, Yamada Y, Narikiyo M, et al. Clinicopathological and prognostic significance of mucin phenotype in gastric cancer. J Surg Oncol. 2008; 98:124–9.
Article5. Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009; 24:193–201.
Article6. Reis CA, David L, Correa P, et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999; 59:1003–7.7. Pyo JS, Kang G, Kim DH, et al. Activation of nuclear factor-kappaB contributes to growth and aggressiveness of papillary thyroid carcinoma. Pathol Res Pract. 2013; 209:228–32.8. Akyürek N, Akyol G, Dursun A, Yamaç D, Günel N. Expression of MUC1 and MUC2 mucins in gastric carcinomas: their relationship with clinicopathologic parameters and prognosis. Pathol Res Pract. 2002; 198:665–74.
Article9. Baldus SE, Zirbes TK, Engel S, et al. Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int J Cancer. 1998; 79:133–8.
Article10. Barresi V, Vitarelli E, Grosso M, Tuccari G, Barresi G. Relationship between immunoexpression of mucin peptide cores MUC1 and MUC2 and Lauren’s histologic subtypes of gastric carcinomas. Eur J Histochem. 2006; 50:301–9.11. Lee HW, Yang DH, Kim HK, et al. Expression of MUC2 in gastric carcinomas and background mucosae. J Gastroenterol Hepatol. 2007; 22:1336–43.
Article12. Shiratsu K, Higuchi K, Nakayama J. Loss of gastric gland mucinspecific O-glycan is associated with progression of differentiatedtype adenocarcinoma of the stomach. Cancer Sci. 2014; 105:126–33.13. Tanaka M, Kitajima Y, Sato S, Miyazaki K. Combined evaluation of mucin antigen and E-cadherin expression may help select patients with gastric cancer suitable for minimally invasive therapy. Br J Surg. 2003; 90:95–101.
Article14. Utsunomiya T, Yonezawa S, Sakamoto H, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998; 4:2605–14.15. Wang RQ, Fang DC. Alterations of MUC1 and MUC3 expression in gastric carcinoma: relevance to patient clinicopathological features. J Clin Pathol. 2003; 56:378–84.
Article16. Zhang HK, Zhang QM, Zhao TH, Li YY, Yi YF. Expression of mucins and E-cadherin in gastric carcinoma and their clinical significance. World J Gastroenterol. 2004; 10:3044–7.
Article17. Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993; 41:1479–85.18. Chang SK, Dohrman AF, Basbaum CB, et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994; 107:28–36.
Article19. Kocer B, Soran A, Kiyak G, et al. Prognostic significance of mucin expression in gastric carcinoma. Dig Dis Sci. 2004; 49:954–64.
Article20. Pyo JS, Ko YS, Kang G, et al. Bile acid induces MUC2 expression and inhibits tumor invasion in gastric carcinomas. J Cancer Res Clin Onco. 2014; Dec. 5. [Epub]. http://dx.doi.org/10.1007/s00432-014-1890-1.
Article21. Li L, Huang PL, Yu XJ, Bu XD. Clinicopathological significance of mucin 2 immuno-histochemical expression in colorectal cancer: a meta-analysis. Chin J Cancer Res. 2012; 24:190–5.
Article22. Gong L, Debruyne PR, Witek M, et al. Bile acids initiate lineage-addicted gastroesophageal tumorigenesis by suppressing the EGF receptor-AKT axis. Clin Transl Sci. 2009; 2:286–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alterations of the Mucin Glycoprotein Expression and Their Relationship with the Pathologic Prognostic Factors in Gastric Carcinoma
- Clinical Significance of MUC2 and MUC5AC Expression in Gastric Adenocarcinomas
- Clinicopathologic correlation with MUC expression in advanced gastric cancer
- Immunohistochemical Study of p53 Gene Protein and bcl-2 Gene Protein Expression in Gastric Adenocarcinoma
- Mucin Expression According to the Progression of Gastric Carcinomas