Clin Endosc.
2015 Nov;48(6):481-490. 10.5946/ce.2015.48.6.481.
Clinical Application of Magnifying Endoscopy with Narrow-Band Imaging in the Stomach
- Affiliations
-
- 1Department of Endoscopy, Fukuoka University Chikushi Hospital, Chikushino, Japan. yao@fukuoka-u.ac.jp
- KMID: 2380404
- DOI: http://doi.org/10.5946/ce.2015.48.6.481
Abstract
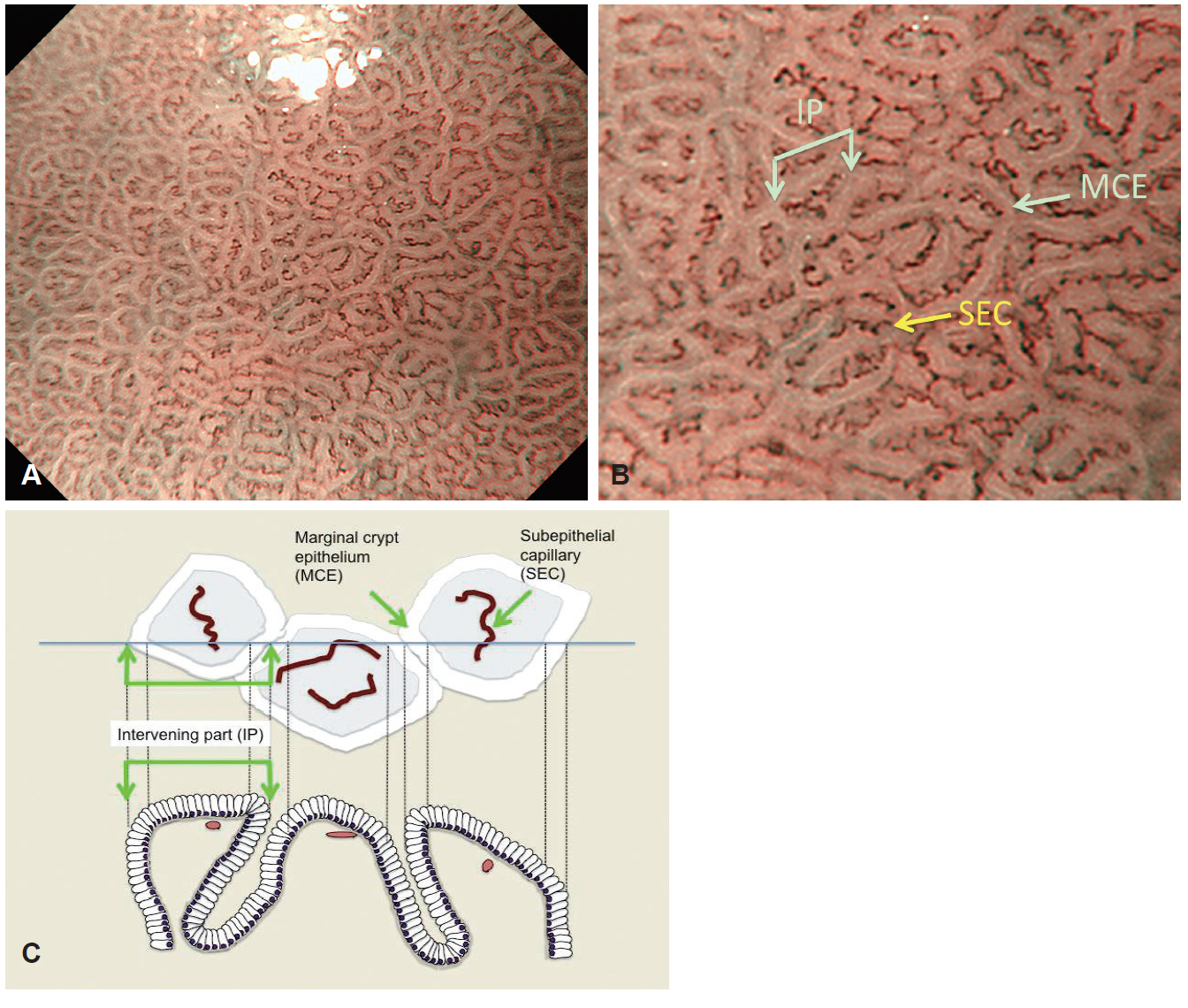
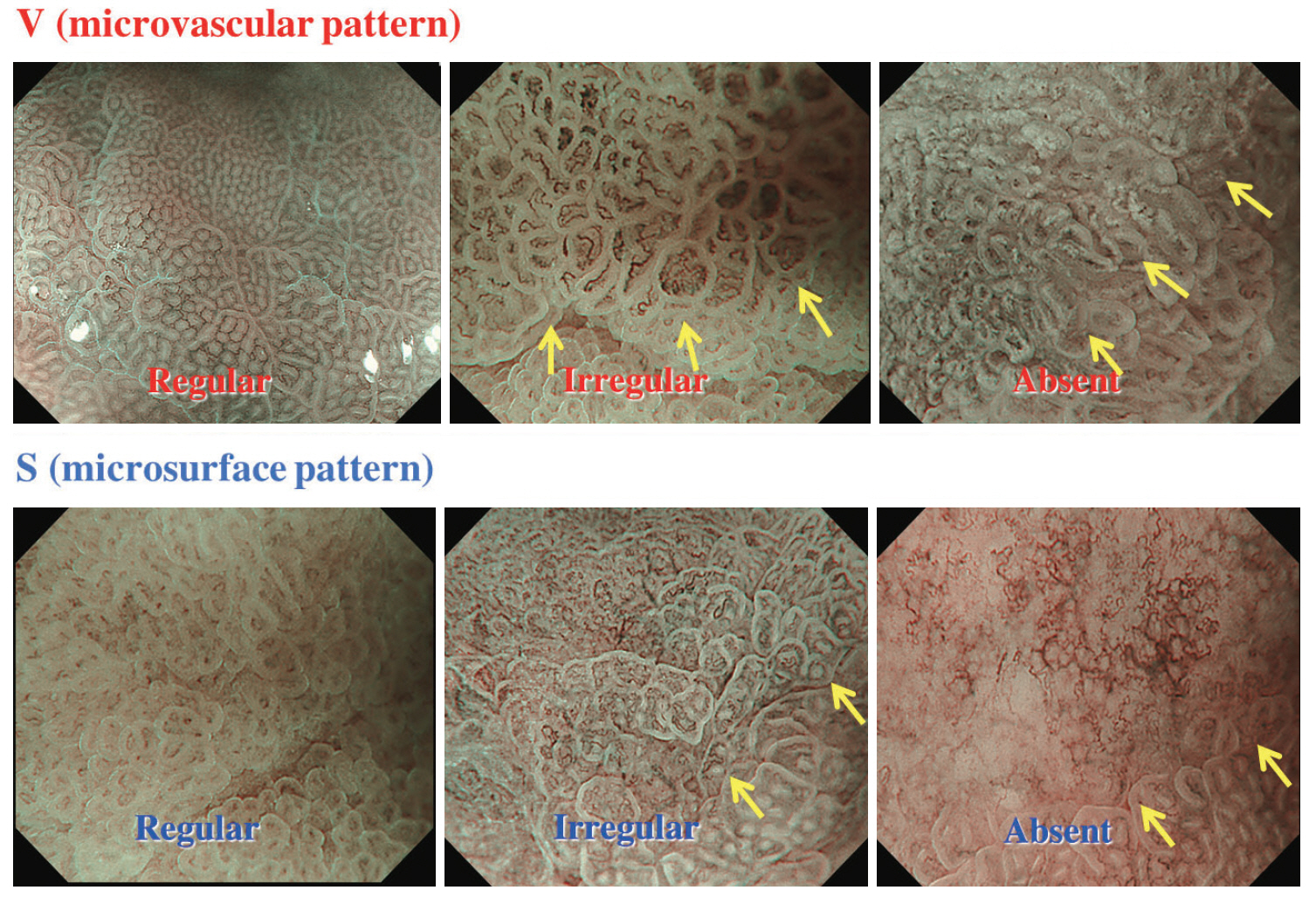
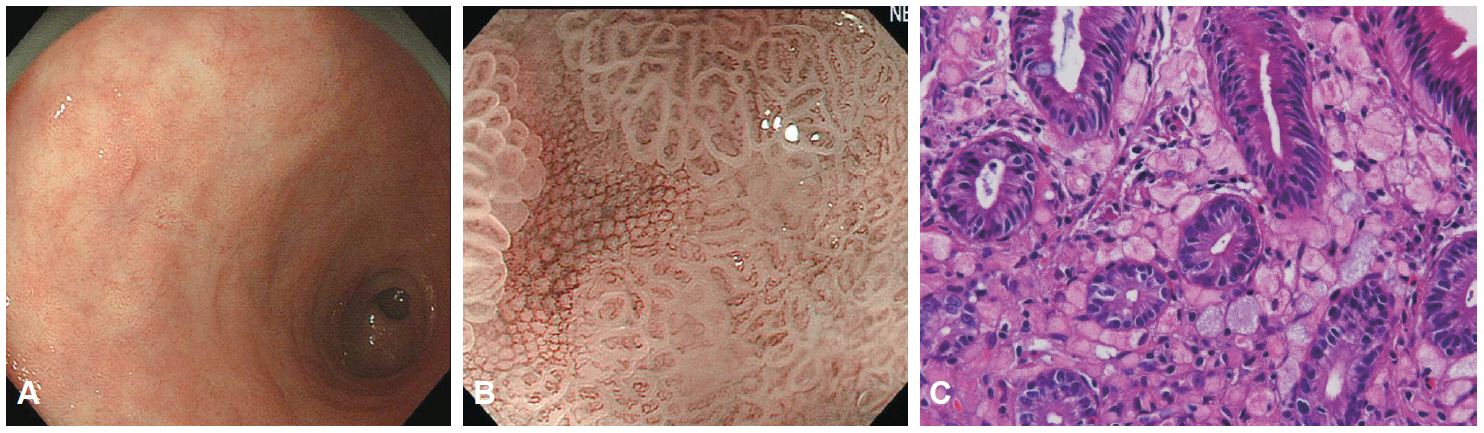
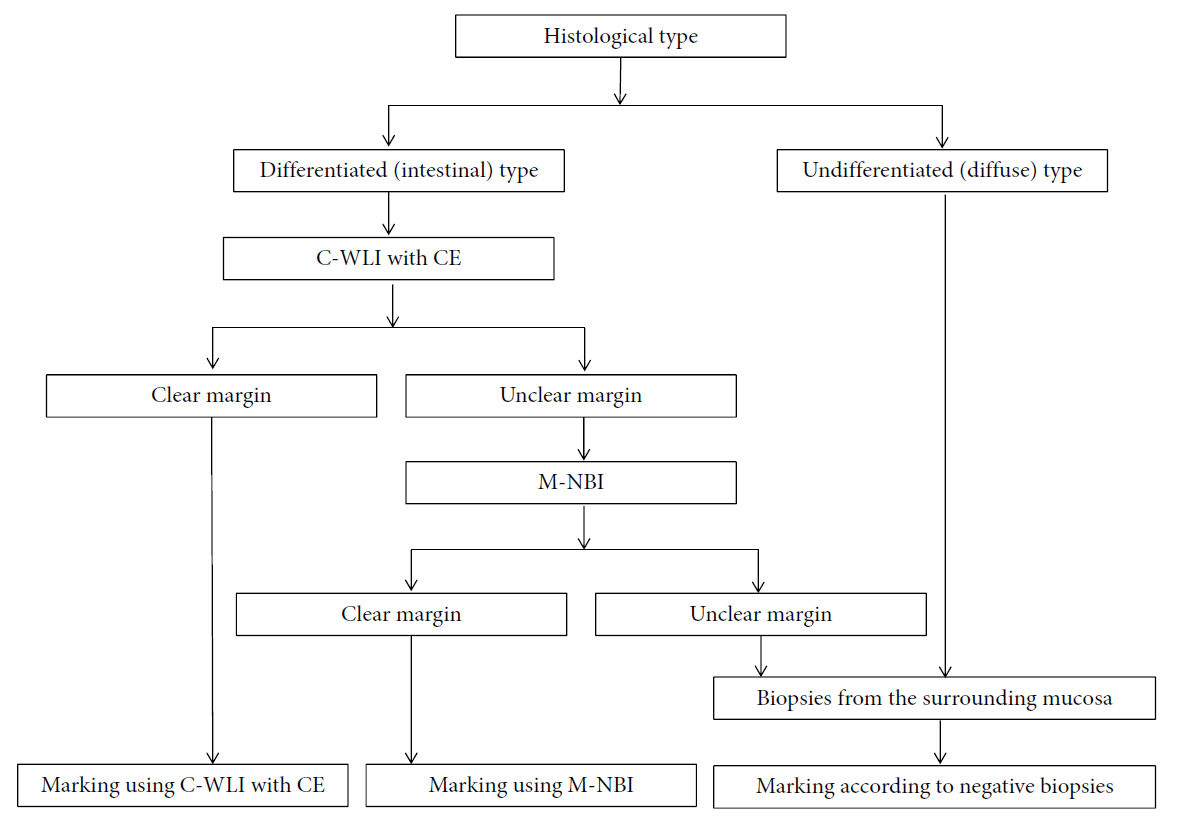
- Magnifying endoscopy with narrow-band imaging (M-NBI) can visualize superficial microanatomies in the stomach. The normal morphology of the microanatomy visualized by M-NBI differs according to the part of the stomach. The gastric fundic glandular mucosa appears as a regular honeycomb-like subepithelial capillary network (SECN) pattern with a regular collecting venule pattern and regular oval crypt opening with circular marginal crypt epithelium (MCE) pattern. The gastric pyloric glandular mucosa displays a regular coil-shaped SECN pattern and regular polygonal or curved MCE pattern. For a diagnosis of early gastric cancer using M-NBI, the vessel plus surface classification system was developed. This system is clinically useful for the differential diagnosis of focal gastritis and small depressed cancer and for determining the horizontal extent of early gastric cancer for successful endoscopic resection. Advantages of M-NBI over conventional endoscopic imaging techniques with white light include accurate diagnosis and cost effectiveness. This technique is a breakthrough in the endoscopic diagnostic field.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Application of Current Image-Enhanced Endoscopy in Gastric Diseases
Wansik Lee
Clin Endosc. 2021;54(4):477-487. doi: 10.5946/ce.2021.160.Multiple White Flat Lesions of the Corpus: Subtype of Hyperplastic Polyps vs. Intestinal Metaplasia
Su Jin Kim, Cheol Woong Choi
Clin Endosc. 2018;51(6):503-504. doi: 10.5946/ce.2018.162.Usefulness of Narrow-Band Imaging in Endoscopic Submucosal Dissection of the Stomach
Jung-Wook Kim
Clin Endosc. 2018;51(6):527-533. doi: 10.5946/ce.2018.186.
Reference
-
1. Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009; 41:462–467.
Article2. Yao K, Iwashita A, Tanabe H, et al. Novel zoom endoscopy technique for diagnosis of small flat gastric cancer: a prospective, blind study. Clin Gastroenterol Hepatol. 2007; 5:869–878.
Article3. Yao K, Oishi T. Microgastroscopic findings of mucosal microvascular architecture as visualized by magnifying endoscopy. Dig Endosc. 2001; 13(Suppl 1):S27–S33.
Article4. Yao K. Microanatomies as visualized using magnifying endoscopy with narrow band imaging in the stomach: which microanatomical structures can we visualize in the glandular epithelium using narrow band imaging, and how is this achieved?. In : Yao K, editor. Zoom Gastroscopy. Tokyo: Springer;2013. p. 57–69.5. Yao K. Clinical applications of magnifying endoscopy with narrow-band imaging (M-NBI) of the stomach. In : Yao K, editor. Zoom Gastroscopy. Tokyo: Springer;2013. p. 83–87.6. Nakagawa S, Kato M, Shimizu Y, et al. Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest Endosc. 2003; 58:71–75.
Article7. Anagnostopoulos GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007; 39:202–207.
Article8. Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006; 38:819–824.
Article9. Yao K, Iwashita A, Tanabe H, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008; 68:574–580.
Article10. Matsushita M, Mori S, Uchida K, Nishio A, Okazaki K. “White opaque substance” and “light blue crest” within gastric flat tumors or intestinal metaplasia: same or different signs? Gastrointest Endosc. 2009; 70:402.
Article11. Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002; 56:279–284.
Article12. Yao K, Iwashita A, Kikuchi Y, et al. Novel zoom endoscopy technique for visualizing the microvascular architecture in gastric mucosa. Clin Gastroenterol Hepatol. 2005; 3(7 Suppl 1):S23–S26.
Article13. Ezoe Y, Muto M, Horimatsu T, et al. Magnifying narrow-band imaging versus magnifying white-light imaging for the differential diagnosis of gastric small depressive lesions: a prospective study. Gastrointest Endosc. 2010; 71:477–484.
Article14. Ezoe Y, Muto M, Uedo N, et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011; 141:2017–2025.
Article15. Miwa K, Doyama H, Ito R, et al. Can magnifying endoscopy with narrow band imaging be useful for low grade adenomas in preoperative biopsy specimens? Gastric Cancer. 2012; 15:170–178.
Article16. Maki S, Yao K, Nagahama T, et al. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between lowgrade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer. 2013; 16:140–146.
Article17. Yao K. How is the VS (vessel plus surface) classification system applicable to magnifying narrow-band imaging examinations of gastric neoplasias initially diagnosed as low-grade adenomas? Gastric Cancer. 2012; 15:118–120.
Article18. Tao G, Xing-Hua L, Ai-Ming Y, et al. Enhanced magnifying endoscopy for differential diagnosis of superficial gastric lesions identified with white-light endoscopy. Gastric Cancer. 2014; 17:122–129.
Article19. Yamada S, Doyama H, Yao K, et al. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging: a post-hoc analysis of a prospective randomized controlled trial. Gastrointest Endosc. 2014; 79:55–63.20. Yao K, Doyama H, Gotoda T, et al. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: a prospective multicenter feasibility study. Gastric Cancer. 2014; 17:669–679.
Article21. Fujiwara S, Yao K, Nagahama T, et al. Can we accurately diagnose minute gastric cancers (</=5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (M-NBI). Gastric Cancer. 2015; 18:590–596.22. Yao K, Yao T, Iwashita A. Determining the horizontal extent of early gastric carcinoma: two modern techniques based on differences in the mucosal microvascular architecture and density between carcinomatous and non-carcinomatous mucosa. Dig Endosc. 2002; 14(Suppl 1):S83–S87.
Article23. Nagahama T, Yao K, Maki S, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011; 74:1259–1267.
Article24. Yao K. The vessels plus surface (VS) classification system in the diagnosis of early gastric cancer. In : Yao K, editor. Zoom Gastroscopy. Tokyo: Springer;2013. p. 89–98.25. Kanesaka T, Sekikawa A, Tsumura T, et al. Dense-type crypt opening seen on magnifying endoscopy with narrow-band imaging is a feature of gastric adenoma. Dig Endosc. 2014; 26:57–62.
Article26. Kanzaki H, Uedo N, Ishihara R, et al. Comprehensive investigation of areae gastricae pattern in gastric corpus using magnifying narrow band imaging endoscopy in patients with chronic atrophic fundic gastritis. Helicobacter. 2012; 17:224–231.
Article27. Kanemitsu T, Yao K, Nagahama T, et al. The vessels within epithelial circle (VEC) pattern as visualized by magnifying endoscopy with narrow-band imaging (ME-NBI) is a useful marker for the diagnosis of papillary adenocarcinoma: a case-controlled study. Gastric Cancer. 2014; 17:469–477.
Article28. Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004; 36:1080–1084.
Article29. Kanesaka T, Sekikawa A, Tsumura T, et al. Absent microsurface pattern is characteristic of early gastric cancer of undifferentiated type: magnifying endoscopy with narrow-band imaging. Gastrointest Endosc. 2014; 80:1194–1198.
Article30. Doyama H, Yoshida N, Tsuyama S, et al. The “white globe appearance” (WGA): a novel marker for a correct diagnosis of early gastric cancer by magnifying endoscopy with narrow-band imaging (M-NBI). Endosc Int Open. 2015; 3:E120–E124.
Article31. Yoshida N, Doyama H, Nakanishi H, et al. White globe appearance is a novel specific endoscopic marker for gastric cancer: a prospective study. Dig Endosc. 2015 Jul 31 [Epub]. http://dx.doi.org/10.1111/den.12519.
Article32. Yao K, Iwashita A, Nambu M, et al. Nature of white opaque substance in gastric epithelial neoplasia as visualized by magnifying endoscopy with narrow-band imaging. Dig Endosc. 2012; 24:419–425.
Article33. Ueo T, Yonemasu H, Yada N, et al. White opaque substance represents an intracytoplasmic accumulation of lipid droplets: immunohistochemical and immunoelectron microscopic investigation of 26 cases. Dig Endosc. 2013; 25:147–155.
Article34. Ueyama H, Matsumoto K, Nagahara A, et al. A white opaque substance-positive gastric hyperplastic polyp with dysplasia. World J Gastroenterol. 2013; 19:4262–4266.
Article35. Ohtsu K, Yao K, Matsunaga K, et al. Lipid is absorbed in the stomach by epithelial neoplasms (adenomas and early cancers): a novel functional endoscopy technique. Endosc Int Open. 2015; 3:E318–E322.
Article36. Enjoji M, Kohjima M, Ohtsu K, et al. Intracellular mechanisms underlying lipid accumulation (white opaque substance) in gastric epithelial neoplasms: a pilot study of expression profiles of lipid-metabolism-associated genes. J Gastroenterol Hepatol. 2015 Oct 29 [Epub]. http://dx.doi.org/10.1111/jgh.13216.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Narrow-Band Imaging in Endoscopic Submucosal Dissection of the Stomach
- Image-Enhanced Endoscopy and Its Corresponding Histopathology in the Stomach
- Clinical Usefulness of Magnifying Chromoendoscopy and Magnifying Narrow Band Imaging Endoscopy for Predicting the Submucosal Invasion of Early Colorectal Cancers
- The Usefulness of Magnifying Endoscopy and Narrow-Band Imaging in Measuring the Depth of Invasion before Endoscopic Submucosal Dissection
- Application of artificial intelligence for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging