Ann Dermatol.
2017 Jun;29(3):276-282. 10.5021/ad.2017.29.3.276.
Therapeutic Efficacy of a Combination Therapy of Topical 17α-Estradiol and Topical Minoxidil on Female Pattern Hair Loss: A Noncomparative, Retrospective Evaluation
- Affiliations
-
- 1Department of Dermatology and Institute of Hair and Cosmetic Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. leewonsoo@yonsei.ac.kr
- KMID: 2378525
- DOI: http://doi.org/10.5021/ad.2017.29.3.276
Abstract
- BACKGROUND
A variety of agents have been used to treat female pattern hair loss (FPHL), including topical minoxidil, topical 17α-estradiol, oral anti-androgen agents, and mineral supplements. Compared with these single agent regimens, combination therapies could be a better therapeutic option in expectation of superior treatment outcome.
OBJECTIVE
This study was designed to determine the efficacy of a combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil in Korean patients with FPHL.
METHODS
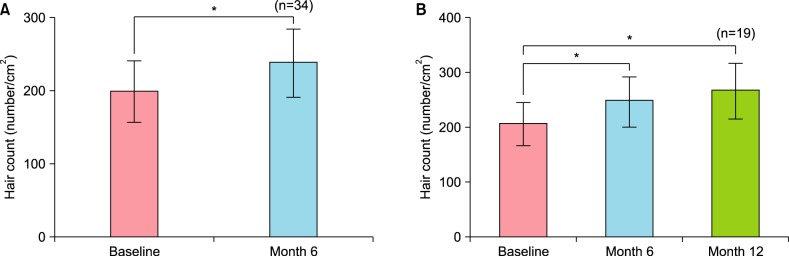
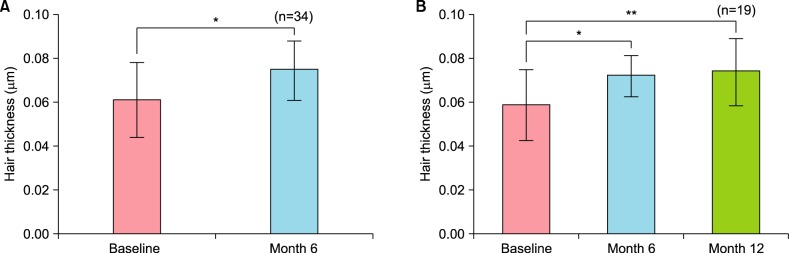
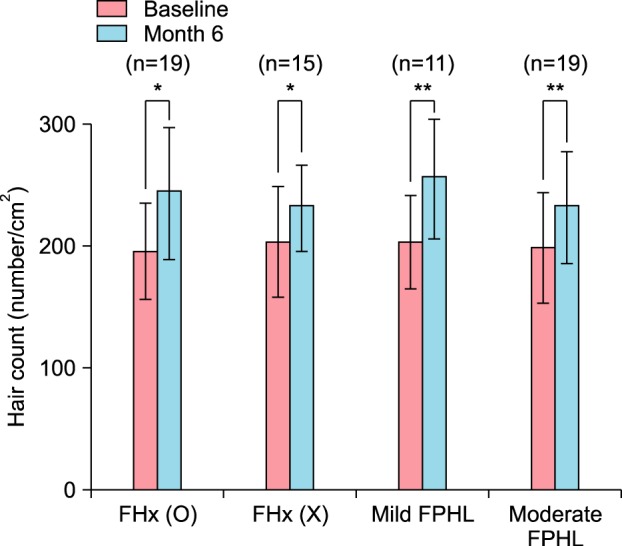
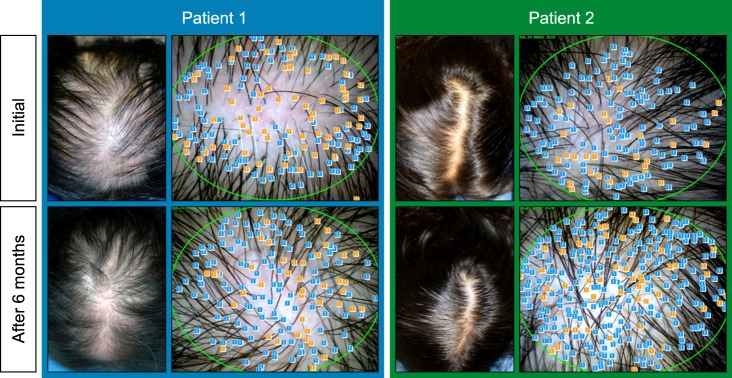
Therapeutic efficacy was evaluated in 34 women who applied topical 0.025% 17α-estradiol and 3% minoxidil once daily for more than 6 months. Phototrichogram analysis was performed before and after therapy. The efficacy was evaluated with respect to total hair count, hair caliber (as assessed by phototrichogram analysis), and photographic assessment.
RESULTS
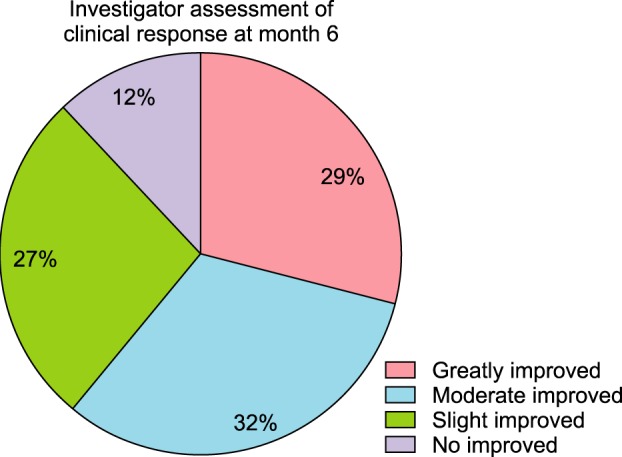
Total hair count and hair caliber both increased from baseline to 6 months in patients treated with the combination therapy of topical 0.025% 17α-estradiol and 3% minoxidil (p<0.001). Photographic assessment also revealed significant disease improvement, thus supporting the therapeutic efficacy.
CONCLUSION
A combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil can be tried as an effective treatment for FPHL.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Ann Dermatol. 2012; 24:243–252. PMID: 22879706.
Article2. Güleç AT, Tanriverdi N, Dürü C, Saray Y, Akçali C. The role of psychological factors in alopecia areata and the impact of the disease on the quality of life. Int J Dermatol. 2004; 43:352–356. PMID: 15117365.
Article3. Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005; 331:951–953. PMID: 16239692.
Article4. Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007; 2:189–199. PMID: 18044135.5. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999; 341:491–497. PMID: 10441606.
Article6. Kim WJ, Song M, Ko HC, Kim BS, Kim MB. Efficacy of finasteride 1.25 mg on female pattern hair loss; pilot study. Ann Dermatol. 2012; 24:370–372. PMID: 22879729.
Article7. Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). J Investig Dermatol Symp Proc. 2003; 8:20–23.
Article8. Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998; 317:865–869. PMID: 9748188.9. Trüeb RM. New and established methods in therapy of hair diseases. Hautarzt. 2003; 54:732–740. PMID: 12942187.10. Park HY, Lee WS, Park J, Kim DW, Ahn SY, Jung YJ, et al. An open label, multi-center clinical trial of topical 5% minoxidil solution for the treatment of male androgenetic alopecia (a phase IV study). Korean J Dermatol. 2009; 47:295–302.11. Lee WS, Ro BI, Hong SP, Bak H, Sim WY, Kim DW, et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007; 57:37–46. PMID: 17467851.12. van Zuuren EJ, Fedorowicz Z, Carter B. Evidence-based treatments for female pattern hair loss: a summary of a Cochrane systematic review. Br J Dermatol. 2012; 167:995–1010. PMID: 23039053.
Article13. Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011; 65:1126–1134.e2. PMID: 21700360.
Article14. Gupta AK, Charrette A. Topical minoxidil: systematic review and meta-analysis of its efficacy in androgenetic alopecia. Skinmed. 2015; 13:185–189. PMID: 26380504.15. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004; 150:186–194. PMID: 14996087.
Article16. Li M, Marubayashi A, Nakaya Y, Fukui K, Arase S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J Invest Dermatol. 2001; 117:1594–1600. PMID: 11886528.17. Colombe L, Vindrios A, Michelet JF, Bernard BA. Prostaglandin metabolism in human hair follicle. Exp Dermatol. 2007; 16:762–769. PMID: 17697149.
Article18. Hoffmann R, Niiyama S, Huth A, Kissling S, Happle R. 17alpha-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Exp Dermatol. 2002; 11:376–380. PMID: 12190948.19. Smart RC, Oh HS. On the effect of estrogen receptor agonists and antagonists on the mouse hair follicle cycle. J Invest Dermatol. 1998; 111:175. PMID: 9665409.20. Whitaker WL, Baker BL. A comparison of the direct action of estrogen and adrenal cortical extracts on growth of hair in the rat. J Invest Dermatol. 1951; 17:69–77. PMID: 14861483.21. Kim JH, Lee SY, Lee HJ, Yoon NY, Lee WS. 17α-Estradiol (Ell-Cranell® alpha 0.025%) solution on female pattern hair loss: single center, open-label, non-comparative, phase IV study. Ann Dermatol. 2012; 24:295–305. PMID: 22879713.22. Orfanos CE, Vogels L. Local therapy of androgenetic alopecia with 17 alpha-estradiol. A controlled, randomized double-blind study (author's transl). Dermatologica. 1980; 161:124–132. PMID: 7398983.23. Wozel G, Naranayan S, Jäckel A, Lutz G. Alfatradiol (0,025%)-Eine wirksame und sichere Therapieoption zur Behandlung der androgenetischen Alopezie bei Frauen und Männern. Akt Dermatol. 2005; 31:553–560.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Generalized Hypertrichosis After Treatment with Topical Minoxidil
- An E-mail Survey of General Awareness and Usage Status of Topical Minoxidil Solution in Alopecia Patient
- The Efficacy and Safety of 17alpha-Estradiol (Ell-Cranell(R) alpha 0.025%) Solution on Female Pattern Hair Loss: Single Center, Open-Label, Non-Comparative, Phase IV Study
- A Case of Allergic Contact Dermatitis Due to Topical Minoxidil
- Hypertrichosis in a Woman During Treatment with 3% Topical Minoxidil