Korean J Neurotrauma.
2015 Oct;11(2):162-166. 10.13004/kjnt.2015.11.2.162.
Spinal Cord Stimulation for Refractory Neuropathic Pain of Neuralgic Amyotrophy
- Affiliations
-
- 1Department of Neurosurgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. sbc@catholic.ac.kr
- 2The Catholic Neuroscience Institute, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2378279
- DOI: http://doi.org/10.13004/kjnt.2015.11.2.162
Abstract
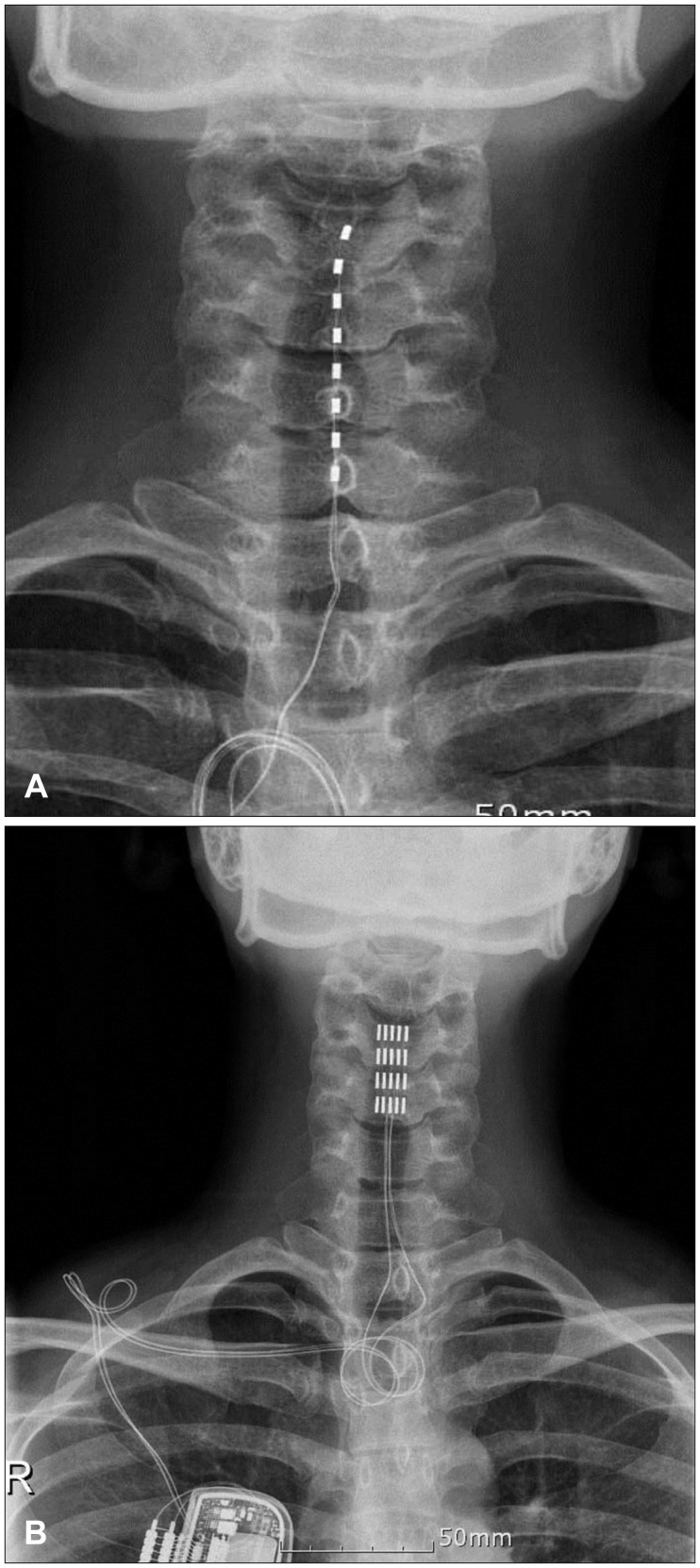
- The aim of this paper was to report the effect of temporary and chronic spinal cord stimulation for refractory neuropathic pain in neuralgic amyotrophy (NA). A 35-year-old female presented with two-months history of a severe, relentless neuropathic pain of the left shoulder, forearm, palm, and fingers. The neuropathic pain was refractory to various medical treatments, including nonsteroidal anti-inflammatory drugs, opiates, epidural and stellate ganglion blocks, and typically unrelenting. The diagnosis of NA was made with the characteristic clinical history and magnetic resonance imaging. The patient underwent a temporary spinal cord stimulation to achieve an adequate pain relief because her pain was notoriously difficult to control and lasted longer than the average duration (about 4 weeks on average) of a painful phase of NA. Permanent stimulation was given with paddle lead. The neuropathic pain in her NA persisted and she continued using the spinal cord stimulation with 12 months after development of NA. The temporary spinal cord stimulation was effective in a patient with an extraordinary prolonged, acute painful phase of NA attack, and the subsequent chronic stimulation was also useful in achieving an adequate analgesia during the chronic phase of NA.
MeSH Terms
Figure
Reference
-
1. Bala MM, Riemsma RP, Nixon J, Kleijnen J. Systematic review of the (cost-)effectiveness of spinal cord stimulation for people with failed back surgery syndrome. Clin J Pain. 2008; 24:741–756. PMID: 18936591.
Article2. Burchiel KJ, Anderson VC, Wilson BJ, Denison DB, Olson KA, Shatin D. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995; 36:1101–1110. discussion 1110-1111PMID: 7643988.
Article3. Cruz-Martínez A, Barrio M, Arpa J. Neuralgic amyotrophy: variable expression in 40 patients. J Peripher Nerv Syst. 2002; 7:198–204. PMID: 12365568.
Article4. Dreschfeld J. On some of the rarer forms of muscular atrophies. Brain. 1886; 9:178–195.
Article5. Geertzen JH, Groothoff JW, Nicolai JP, Rietman JS. Brachial plexus neuropathy. A long-term outcome study. J Hand Surg Br. 2000; 25:461–464. PMID: 10991813.
Article6. Grabow TS, Tella PK, Raja SN. Spinal cord stimulation for complex regional pain syndrome: an evidence-based medicine review of the literature. Clin J Pain. 2003; 19:371–383. PMID: 14600537.
Article7. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007; 132:179–188. PMID: 17845835.
Article8. Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. ed 2. Seattle, WA: IASP Press;1994.9. Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006; 31(4 Suppl):S6–S12. PMID: 16647596.
Article10. Moriyama K. Effect of temporary spinal cord stimulation on postherpetic neuralgia in the thoracic nerve area. Neuromodulation. 2009; 12:39–43. PMID: 22151221.
Article11. Sathasivam S, Lecky B, Manohar R, Selvan A. Neuralgic amyotrophy. J Bone Joint Surg Br. 2008; 90:550–553. PMID: 18450616.
Article12. Son BC, Kim DR, Lee SW, Chough CK. Factors associated with the success of trial spinal cord stimulation in patients with chronic pain from failed back surgery syndrome. J Korean Neurosurg Soc. 2013; 54:501–506. PMID: 24527193.
Article13. Stutz CM. Neuralgic amyotrophy: Parsonage-Turner Syndrome. J Hand Surg Am. 2010; 35:2104–2106. PMID: 21035964.
Article14. Suarez GA, Giannini C, Bosch EP, Barohn RJ, Wodak J, Ebeling P, et al. Immune brachial plexus neuropathy: suggestive evidence for an inflammatory-immune pathogenesis. Neurology. 1996; 46:559–561. PMID: 8614534.
Article15. Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006; 10:91–101. PMID: 16310712.
Article16. Tonali P, Uncini A, Di Pasqua PG. So-called neuralgic amyotrophy: clinical features and long term follow-up. Ital J Neurol Sci. 1983; 4:431–437. PMID: 6674243.
Article17. van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat Rev Neurol. 2011; 7:315–322. PMID: 21556032.
Article18. van Alfen N. The neuralgic amyotrophy consultation. J Neurol. 2007; 254:695–704. PMID: 17446996.
Article19. van Alfen N. The trouble with neuralgic amyotrophy. Pract Neurol. 2006; 6:298–307.
Article20. van Alfen N, van der Werf SP, van Engelen BG. Long-term pain, fatigue, and impairment in neuralgic amyotrophy. Arch Phys Med Rehabil. 2009; 90:435–439. PMID: 19254608.
Article21. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006; 129(Pt 2):438–450. PMID: 16371410.
Article22. van Alfen N, van Engelen BG, Hughes RA. Treatment for idiopathic and hereditary neuralgic amyotrophy (brachial neuritis). Cochrane Database Syst Rev. 2009; (3):CD006976. PMID: 19588414.
Article23. van Alfen N, van Engelen BG, Reinders JW, Kremer H, Gabreëls FJ. The natural history of hereditary neuralgic amyotrophy in the Dutch population: two distinct types? Brain. 2000; 123(Pt 4):718–723. PMID: 10734003.
Article24. van Eijs F, Smits H, Geurts JW, Kessels AG, Kemler MA, van Kleef M, et al. Brush-evoked allodynia predicts outcome of spinal cord stimulation in complex regional pain syndrome type 1. Eur J Pain. 2010; 14:164–169. PMID: 19942463.
Article25. Vriesendorp FJ, Dmytrenko GS, Dietrich T, Koski CL. Anti-peripheral nerve myelin antibodies and terminal activation products of complement in serum of patients with acute brachial plexus neuropathy. Arch Neurol. 1993; 50:1301–1303. PMID: 8257306.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Posterior Cord Syndrome After Spinal Cord Stimulation Electrode Lead Insertion: A Case Report
- Spinal cord stimulation for neuropathic pain following idiopathic transverse myelitis: A case report
- Spinal Cord Stimulation for the Neuropathic Pain Caused by Traumatic Lumbosacral Plexopathy after Extensive Pelvic Fracture
- Spinal cord stimulation for a patient with neuropathic pain related to congenital syringomyelia
- Spinal Cord Stimulation for Refractory Angina Pectoris: A Case Report