Retrospective Analysis on the Effects of House Dust Mite Specific Immunotherapy for More Than 3 Years in Atopic Dermatitis
- Affiliations
-
- 1Department of Dermatology, Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. kwanglee@yuhs.ac
- KMID: 2374045
- DOI: http://doi.org/10.3349/ymj.2016.57.2.393
Abstract
- PURPOSE
In extrinsic atopic dermatitis (AD), house dust mites (HDM) play a role in eliciting or aggravating allergic lesions. The nature of skin inflammation in AD has raised a growing interest in allergen-specific immunotherapy (SIT). Thus, we assessed clinical improvement and laboratory parameters for evaluation of the benefit of long-term SIT.
MATERIALS AND METHODS
A total of 217 AD patients who were treated with SIT for at least 3 years were retrospectively assessed, by using their investigator global assessment, pruritus scores, loss of sleep (LOS), total serum IgE, and eosinophil counts collected. Patients were additionally classified into subgroups according to age, initial AD severity and mono- or multi-sensitization to include different individual factors in the evaluation of SIT efficacy. Lastly, we compared laboratory data of good responders to SIT with that of poor responders to SIT.
RESULTS
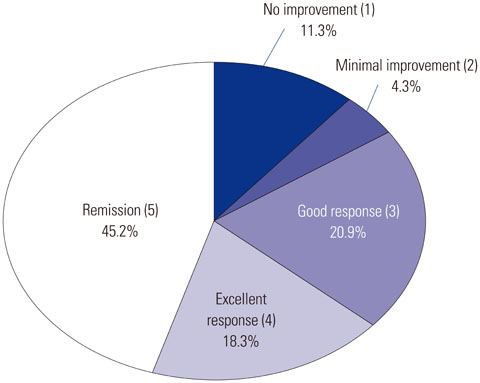
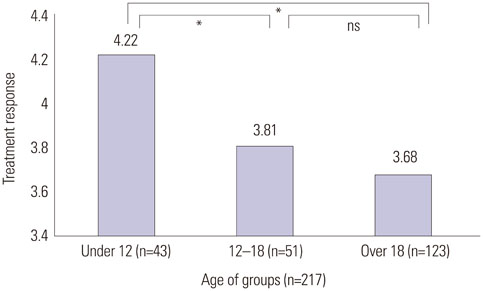
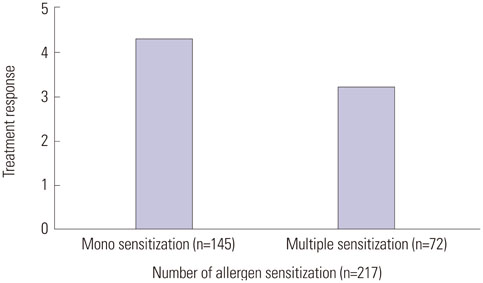
Improvement after SIT therapy was observed in 192 out of 217 patients (88.4%). Among these patients, 138 (63.5%) achieved excellent, near-complete or complete clinical remission. Significant reduction of pruritus, LOS, and the mean value of total serum IgE were observed (p<0.01). Better outcome was found in patients younger than 12 years of age (p=0.024). Patients with moderate to severe AD showed better treatment outcomes (p=0.036). Patients sensitized only to HDM had the better response to treatment, but SIT was also effective in multi-sensitized groups (p=1.051). No significant differences in baseline laboratory results were observed between good and poor responders (p>0.05).
CONCLUSION
We emphasize the usefulness of long-term HDM SIT as a disease-modifying therapy for AD.
MeSH Terms
Figure
Cited by 4 articles
-
Different Responses in Induction of Allergen Specific Immunoglobulin G4 and IgE-Blocking Factors for Three Mite Subcutaneous Immunotherapy Products
Kyung Hee Park, Sang Chul Lee, Young Woong Son, Kyoung Yong Jeong, Yoo Seob Shin, Jung U Shin, Da Woon Sim, Hye Jung Park, Jae-Hyun Lee, Kwang Hoon Lee, Jung-Won Park
Yonsei Med J. 2016;57(6):1427-1434. doi: 10.3349/ymj.2016.57.6.1427.Clinical Efficacy of Subcutaneous Allergen Immunotherapy in Patients with Atopic Dermatitis
Dong-Ho Nahm, Myoung-Eun Kim, Byul Kwon, Su-Mi Cho, Areum Ahn
Yonsei Med J. 2016;57(6):1420-1426. doi: 10.3349/ymj.2016.57.6.1420.Current Status of Patient Education in the Management of Atopic Dermatitis in Korea
Min Kyung Lee, Ju-Hee Seo, Howard Chu, Hyunjung Kim, Yong Hyun Jang, Jae Won Jeong, Hye Yung Yum, Man Yong Han, Ho Joo Yoon, Sang-Heon Cho, Yeong Ho Rha, Jin-Tack Kim, Young Lip Park, Seong Jun Seo, Kwang Hoon Lee, Chang Ook Park
Yonsei Med J. 2019;60(7):694-699. doi: 10.3349/ymj.2019.60.7.694.Safety of Ultra-rush Schedule of Subcutaneous Allergen Immunotherapy With House Dust Mite Extract Conducted in an Outpatient Clinic in Patients With Atopic Dermatitis and Allergic Rhinitis
So-Hee Lee, Myoung-Eun Kim, Yoo Seob Shin, Young-Min Ye, Hae-Sim Park, Dong-Ho Nahm
Allergy Asthma Immunol Res. 2019;11(6):846-855. doi: 10.4168/aair.2019.11.6.846.
Reference
-
1. Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015; 7:221–229.
Article2. Silverberg JI. Atopic dermatitis: an evidence-based treatment update. Am J Clin Dermatol. 2014; 15:149–164.
Article3. Bussmann C, Böckenhoff A, Henke H, Werfel T, Novak N. Does allergen-specific immunotherapy represent a therapeutic option for patients with atopic dermatitis? J Allergy Clin Immunol. 2006; 118:1292–1298.
Article4. Hur GY, Kim TB, Han MY, Nahm DH, Park JW. Allergen and Immunotherapy Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI). A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013; 5:277–282.
Article5. Creticos PS, Adkinson NF Jr, Kagey-Sobotka A, Proud D, Meier HL, Naclerio RM, et al. Nasal challenge with ragweed pollen in hay fever patients. Effect of immunotherapy. J Clin Invest. 1985; 76:2247–2253.
Article6. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102(4 Pt 1):558–562.
Article7. Nahm DH, Cho SM, Kim ME, Kim YJ, Jeon SY. Autologous immunoglobulin therapy in patients with severe recalcitrant atopic dermatitis: a preliminary report. Allergy Asthma Immunol Res. 2014; 6:89–94.
Article8. Warner JO, Price JF, Soothill JF, Hey EN. Controlled trial of hyposensitisation to Dermatophagoides pteronyssinus in children with asthma. Lancet. 1978; 2:912–915.9. Glover MT, Atherton DJ. A double-blind controlled trial of hyposensitization to Dermatophagoides pteronyssinus in children with atopic eczema. Clin Exp Allergy. 1992; 22:440–446.
Article10. Leroy BP, Boden G, Lachapelle JM, Jacquemin MG, Saint-Remy JM. A novel therapy for atopic dermatitis with allergen-antibody complexes: a double-blind, placebo-controlled study. J Am Acad Dermatol. 1993; 28(2 Pt 1):232–239.
Article11. Darsow U, Forer I, Ring J. Allergen-specific immunotherapy in atopic eczema. Curr Allergy Asthma Rep. 2011; 11:277–283.
Article12. Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003; 111:1255–1261.
Article13. Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998; 102:98–106.
Article14. James LK, Durham SR. Update on mechanisms of allergen injection immunotherapy. Clin Exp Allergy. 2008; 38:1074–1088.
Article15. Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003; 112:915–922.
Article16. van Neerven RJ, Arvidsson M, Ipsen H, Sparholt SH, Rak S, Würtzen PA. A double-blind, placebo-controlled birch allergy vaccination study: inhibition of CD23-mediated serum-immunoglobulin E-facilitated allergen presentation. Clin Exp Allergy. 2004; 34:420–428.
Article17. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013; 132:110–117.
Article18. Wüthrich B. [Atopic neurodermatitis]. Wien Med Wochenschr. 1989; 139:156–165.19. Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013; 68:498–506.
Article20. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006; 38:441–446.
Article21. Weidinger S, Rodríguez E, Stahl C, Wagenpfeil S, Klopp N, Illig T, et al. Filaggrin mutations strongly predispose to early-onset and extrinsic atopic dermatitis. J Invest Dermatol. 2007; 127:724–726.
Article22. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011; 365:1315–1327.
Article23. Kezic S, O'Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011; 66:934–940.
Article24. Thyssen JP, Linneberg A, Johansen JD, Carlsen BC, Zachariae C, Meldgaard M, et al. Atopic diseases by filaggrin mutations and birth year. Allergy. 2012; 67:705–708.
Article25. Novak N, Bieber T, Hoffmann M, Fölster-Holst R, Homey B, Werfel T, et al. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol. 2012; 130:925–931.e4.
Article26. Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol. 2010; 11:351–361.27. Ferreira F, Wolf M, Wallner M. Molecular approach to allergy diagnosis and therapy. Yonsei Med J. 2014; 55:839–852.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex
- Patch test and Specific IgE Concentration with House Dust Mite Antigens in Atopic Dermatitis Patients
- Food and house dust mite allergens in children with atopic dermatitis
- Correlation between House Dust Mite Allergen Concentrations in Scalp Dander and Clinical Severity of Atopic Dermatitis in Children
- Allergen-specific Immunotherapy in Patients with Atopic Dermatitis