Korean J Ophthalmol.
2016 Dec;30(6):399-409. 10.3341/kjo.2016.30.6.399.
Epiretinal Proliferation Associated with Macular Hole and Intraoperative Perifoveal Crown Phenomenon
- Affiliations
-
- 1Department of Ophthalmology, HanGil Eye Hospital, Incheon, Korea. jhsohn19@hanafos.com
- 2Department of Pathology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- KMID: 2373997
- DOI: http://doi.org/10.3341/kjo.2016.30.6.399
Abstract
- PURPOSE
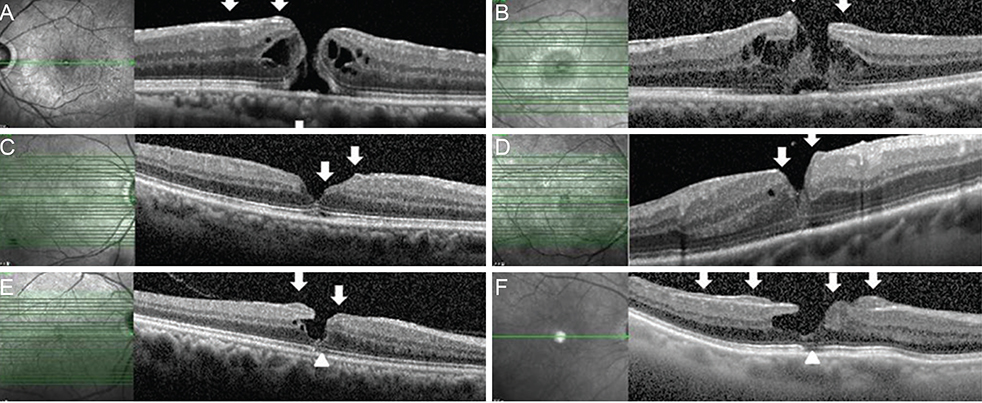
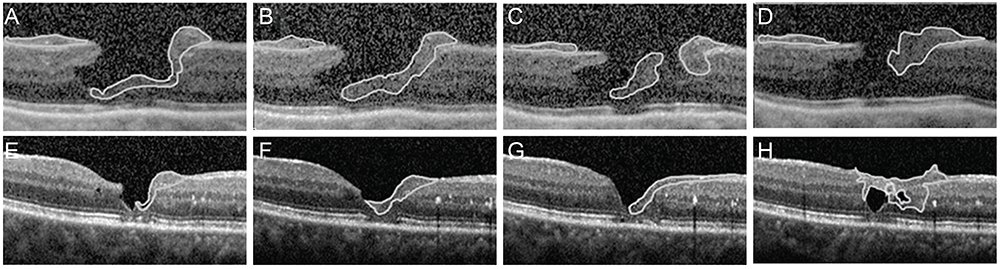
To discuss the unique morphology and origin of epiretinal proliferation associated with macular hole (EPMH) occasionally observed in full-thickness macular hole (FT-MH) or lamellar hole (LH) and to introduce the perifoveal crown phenomenon encountered when removing this unusual proliferative tissue.
METHODS
Sixteen patients showing EPMH in spectral domain-optical coherence tomography were selected from 212 patients diagnosed with MH, LH, FT-MH, impending MH, macular pseudohole, or epiretinal membrane between January 2013 and December 2014. Of the 212 patients included for clinical analysis, 33, 23, 11, 7, and 190 exhibited LH, FT-MH, impending MH, macular pseudohole, and epiretinal membrane, respectively. We reviewed visual acuity, macular morphology, and clinical course. Surgical specimens were analyzed histologically.
RESULTS
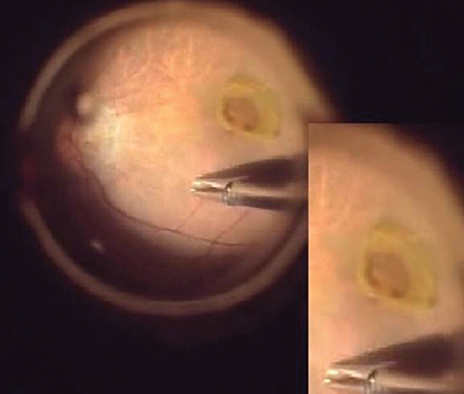
EPMH presented as an amorphous proliferation starting from the defective inner/outer segment (IS/OS) junction covering the inner macula surface. Among the 16 patients with EPMH, 11 underwent vitrectomy, and all exhibited the intraoperative perifoveal crown phenomenon. EPMH tissue was sampled in three patients, one of whom had more tissue removed than intended and showed delayed recovery in visual acuity. Despite hole closure, IS/OS junction integrity was not successfully restored in four of 11 patients. Five patients were followed-up without surgical intervention. Visual acuity slightly decreased in three patients and did not change in one patient, while the remaining patient was lost during follow-up. Among the three perifoveal crown tissues obtained, two were successfully analyzed histologically. Neither tissue showed positivity to synaptophysin or S-100 protein, but one showed positivity to cytokeratin protein immunohistochemical staining.
CONCLUSIONS
EPMH exhibited a distinct but common configuration in spectral domain-optical coherence tomography. An epithelial proliferation origin is plausible based on its configuration and histological analysis. Perifoveal crown phenomenon was observed when removing EPMH during vitrectomy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Epiretinal Proliferation Associated with Lamellar Hole or Macular Hole: Origin and Surgical Prognosis
Young Seong Yang, Ji Shin Lee, Gisung Son, Joonhong Sohn
Korean J Ophthalmol. 2019;33(2):142-149. doi: 10.3341/kjo.2018.0070.
Reference
-
1. Tanner V, Chauhan DS, Jackson TL, Williamson TH. Optical coherence tomography of the vitreoretinal interface in macular hole formation. Br J Ophthalmol. 2001; 85:1092–1097.2. Haouchine B, Massin P, Tadayoni R, et al. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol. 2004; 138:732–739.3. Gaudric A, Haouchine B, Massin P, et al. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol. 1999; 117:744–751.4. Takahashi H, Kishi S. Tomographic features of a lamellar macular hole formation and a lamellar hole that progressed to a full-thickness macular hole. Am J Ophthalmol. 2000; 130:677–679.5. Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995; 102:217–229.6. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995; 102:748–756.7. Tsujikawa M, Ohji M, Fujikado T, et al. Differentiating full thickness macular holes from impending macular holes and macular pseudoholes. Br J Ophthalmol. 1997; 81:117–122.8. Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006; 113:388–397.9. Parolini B, Schumann RG, Cereda MG, et al. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Invest Ophthalmol Vis Sci. 2011; 52:9074–9083.10. Michalewska Z, Michalewski J, Odrobina D, Nawrocki J. Non-full-thickness macular holes reassessed with spectral domain optical coherence tomography. Retina. 2012; 32:922–929.11. Bottoni F, Deiro AP, Giani A, et al. The natural history of lamellar macular holes: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2013; 251:467–475.12. Schumann RG, Compera D, Schaumberger MM, et al. Epiretinal membrane characteristics correlate with photoreceptor layer defects in lamellar macular holes and macular pseudoholes. Retina. 2015; 35:727–735.13. Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014; 34:1513–1523.14. Pang CE, Spaide RF, Freund KB. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015; 35:720–726.15. Compera D, Entchev E, Haritoglou C, et al. Lamellar hole-associated epiretinal proliferation in comparison to epiretinal membranes of macular pseudoholes. Am J Ophthalmol. 2015; 160:373–384.e1.16. Shiraga F, Takasu I, Fukuda K, et al. Modified vitreous surgery for symptomatic lamellar macular hole with epiretinal membrane containing macular pigment. Retina. 2013; 33:1263–1269.17. Heidenkummer HP, Kampik A. Morphologic analysis of epiretinal membranes in surgically treated idiopathic macular foramina: results of light and electron microscopy. Ophthalmologe. 1996; 93:675–679.18. Kampik A, Kenyon KR, Michels RG, et al. Epiretinal and vitreous membranes: comparative study of 56 cases. Arch Ophthalmol. 1981; 99:1445–1454.19. Schumann RG, Schaumberger MM, Rohleder M, et al. Ultrastructure of the vitreomacular interface in full-thickness idiopathic macular holes: a consecutive analysis of 100 cases. Am J Ophthalmol. 2006; 141:1112–1119.20. Fano G, Biocca S, Fulle S, et al. The S-100: a protein family in search of a function. Prog Neurobiol. 1995; 46:71–82.21. Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25:397–424.22. Bignami A, Dahl D. The radial glia of Muller in the rat retina and their response to injury: an immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979; 28:63–69.23. Eisenfeld AJ, Bunt-Milam AH, Sarthy PV. Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984; 25:1321–1328.24. Terenghi G, Cocchia D, Michetti F, et al. Localization of S-100 protein in Muller cells of the retina-1: light microscopical immunocytochemistry. Invest Ophthalmol Vis Sci. 1983; 24:976–980.25. Cocchia D, Polak JM, Terenghi G, et al. Localization of S-100 protein in Muller cells of the retina-2: electron microscopical immunocytochemistry. Invest Ophthalmol Vis Sci. 1983; 24:980–984.26. Lewis GP, Chapin EA, Luna G, et al. The fate of Muller's glia following experimental retinal detachment: nuclear migration, cell division, and subretinal glial scar formation. Mol Vis. 2010; 16:1361–1372.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Spontaneous Closure of Macular Hole in a Previously Vitrectomized Eye
- The Relation between Idiopathic Macular Hole and Perifoveal Vitreous Detachment
- Spontaneous Disappearance of A Traumatic Macular Hole
- Prevalence and Progression of Stage 0 Macular Hole in Fellow Eyes of Patients with Idiopathic Full-thickness Macular Hole
- Epiretinal Proliferation Associated with Lamellar Hole or Macular Hole: Origin and Surgical Prognosis