Ann Lab Med.
2016 Sep;36(5):413-419. 10.3343/alm.2016.36.5.413.
Performance Evaluation of the Serum Thyroglobulin Assays With Immunochemiluminometric Assay and Immunoradiometric Assay for Differentiated Thyroid Cancer
- Affiliations
-
- 1Division of Endocrinology & Metabolism, Department of Medicine, Thyroid Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. swkimmd@skku.edu
- 2Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. nayadoo@hanmail.net
- KMID: 2373570
- DOI: http://doi.org/10.3343/alm.2016.36.5.413
Abstract
- BACKGROUND
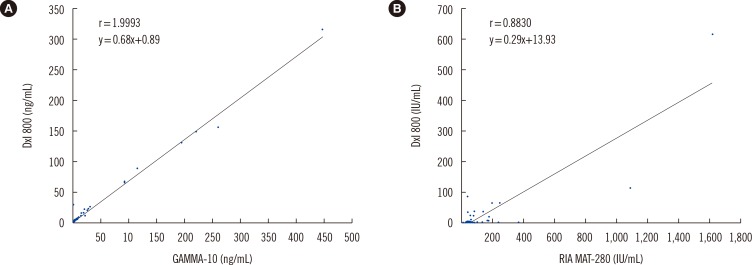
Measurement of postoperative serum thyroglobulin (Tg) is important for detecting persistent or recurrent differentiated thyroid cancer. We evaluated the analytic performance of the DxI 800 assay (Beckman Coulter, USA) for serum Tg and anti-thyroglobulin antibodies (TgAbs) in comparison with that of the GAMMA-10 assay (Shinjin Medics Inc., Korea) for serum Tg and RIA-MAT 280 assay (Stratec, Germany) for TgAb.
METHODS
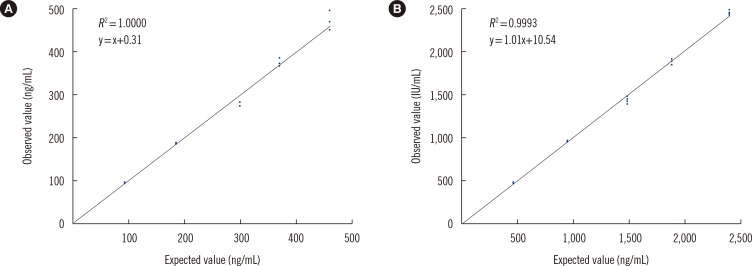
We prospectively collected blood samples from 99 patients thyroidectomized for thyroid cancer. The functional sensitivity was investigated in standards and human serum. Precision and linearity were evaluated according to the guidelines of the Clinical and Laboratory Standards Institute. The correlation between the two assays was assessed in samples with different Tg ranges.
RESULTS
The functional sensitivity of the DxI 800 assay for serum Tg was between 0.0313 and 0.0625 ng/mL. The total CV was 3.9-5.6% for serum Tg and 5.3-6.9% for serum TgAb. The coefficient of determination (R2) was 1.0 and 0.99 for serum Tg and TgAb, respectively. The cut-offs for serum TgAb were 4.0 IU/mL (DxI 800) and 60.0 IU/mL (RIA-MAT 280), and the overall agreement was 68.7%. The correlation between the two assays was excellent; the correlation coefficient was 0.99 and 0.88 for serum Tg and TgAb, respectively.
CONCLUSIONS
The DxI 800 is a sensitive assay for serum Tg and TgAb, and the results correlated well with those from the immunoradiometric assays (IRMA). This assay has several advantages over the IRMA and could be considered an alternative test for Tg measurement.
MeSH Terms
Figure
Reference
-
1. Van Herle AJ, Vassart G, Dumont JE. Control of thyroglobulin synthesis and secretion (second of two parts). N Engl J Med. 1979; 301:307–314. PMID: 88013.2. Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012; 97:2754–2763. PMID: 22639291.
Article3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.
Article4. Van Herle AJ, Uller RP, Matthews NI, Brown J. Radioimmunoassay for measurement of thyroglobulin in human serum. J Clin Invest. 1973; 52:1320–1327. PMID: 4739914.
Article5. Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005; 90:5566–5575. PMID: 15985472.
Article6. Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyroglobulin assays. Clin Chem. 1996; 42:164–173. PMID: 8565221.7. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003; 13:3–126. PMID: 12625976.8. Görges R, Maniecki M, Jentzen W, Sheu SN, Mann K, Bockisch A, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005; 153:49–55. PMID: 15994745.9. Spencer CA, Takeuchi M, Kazarosyan M, Wang CC, Guttler RB, Singer PA, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998; 83:1121–1127. PMID: 9543128.10. Netzel BC, Grebe SK, Carranza Leon BG, Castro MR, Clark PM, Hoofnagle AN, et al. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays and correlation of results with clinical outcomes. J Clin Endocrinol Metab. 2015; 100:E1074–E1083. PMID: 26079778.11. Persoon AC, Van Den Ouweland JM, Wilde J, Kema IP, Wolffenbuttel BH, Links TP. Clinical utility of an automated immunochemiluminometric thyroglobulin assay in differentiated thyroid carcinoma. Clin Chem. 2006; 52:686–691. PMID: 16595825.12. Spencer C, Petrovic I, Fatemi S, LoPresti J. Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by falsenegative and false-positive serum thyroglobulin autoantibody misclassifications. J Clin Endocrinol Metab. 2014; 99:4589–4599. PMID: 25226290.13. Lee JI, Kim JY, Choi JY, Kim HK, Jang HW, Hur KY, et al. Differences in serum thyroglobulin measurements by 3 commercial immunoradiometric assay kits and laboratory standardization using Certified Reference Material 457 (CRM-457). Head Neck. 2010; 32:1161–1166. PMID: 20029980.14. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.15. Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guidelinesecond ed. CLSI document EP5-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2004.16. Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS, Kim SC, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005; 90:1440–1445. PMID: 15613412.17. Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88:1433–1441. PMID: 12679418.18. Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods -Strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2013; 27:701–712. PMID: 24094640.19. Kim WG, Yoon JH, Kim WB, Kim TY, Kim EY, Kim JM, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008; 93:4683–4689. PMID: 18812478.20. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214. PMID: 19860577.21. Preissner CM, O'Kane DJ, Singh RJ, Morris JC, Grebe SK. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab. 2003; 88:3069–3074. PMID: 12843145.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum Thyroglobulin Concentrations in Congenital Hypothyroidism
- Effects of Anti-thyroglobulin Antibody on the Measurement of Thyroglobulin: Differences Between Immunoradiometric Assay Kits Available
- Postoperative Follow-Up of Differentiated Thyroid Cancer: Use of Thyroglobulin Assay
- Influence of Anti-thyroglobulin Antibody on the Measurement of Thyroglobulin using the Immunoradiometric Assay
- Significant Issues Derived from the Choice of a PSA Test for Measuring PSA in Serum: Comparison of IMx Enzyme immunoassay and ELSA Immunoradiometric Assay