Ann Lab Med.
2016 Jul;36(4):335-341. 10.3343/alm.2016.36.4.335.
Serious Adverse Transfusion Reactions Reported in the National Recipient-Triggered Trace Back System in Korea (2006-2014)
- Affiliations
-
- 1Division of Human Blood Safety Surveillance, Korea Centers for Disease Control & Prevention, Cheongju, Korea.
- 2Department of Laboratory Medicine, Chonnam National University Hospital, Gwangju, Korea.
- 3Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea.
- 6Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunwan University School of Medicine, Seoul, Korea. ldhmd@korea.kr duck.cho@samsung.com
- 7Division of Infectious Disease Surveillance, Korea Centers for Disease Control & Prevention, Cheongju, Korea. ldhmd@korea.kr duck.cho@samsung.com
- KMID: 2373553
- DOI: http://doi.org/10.3343/alm.2016.36.4.335
Abstract
- BACKGROUND
Adverse transfusion reactions (ATRs) are clinically relevant to patients with significant morbidity and mortality. This study aimed to review the cases of ATR reported in the recipient-triggered trace back system for a recent nine-year period in Korea.
METHODS
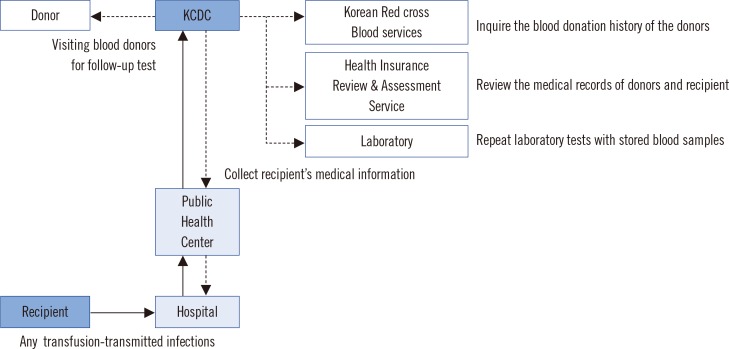
Nine-year data obtained from 2006 to 2014 by the trace back system at the Division of Human Blood Safety Surveillance of the Korean Centers for Disease Control (KCDC) were reviewed. The suspected cases were assessed according to six categories: (i) related to, (ii) probably related to, (iii) probably not related to, (iv) not related to transfusion, (v) unable to investigate, and (vi) under investigation.
RESULTS
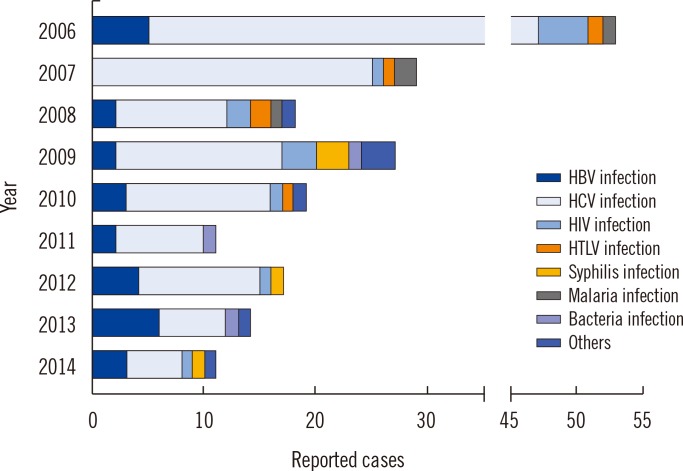
Since 2006, 199 suspected serious ATRs were reported in hospitals and medical institutions in Korea, and these ATRs were reassessed by the division of Human Blood Safety Surveillance of the KCDC. Among the reported 193 cases as transfusion related infections, hepatitis C virus (HCV) infection (135, 67.8%) was reported most frequently, followed by hepatitis B virus (HBV) infection (27, 13.6%), HIV infection (13, 6.5%), syphilis (9, 4.5%), malarial infection (4, 2.0%), other bacterial infections (3, 1.5%), HTLV infection (1, 0.5%), and scrub typhus infection (1, 0.5%), respectively. Of the 199 cases, 13 (6.5%) cases were confirmed as transfusion-related (3 HCV infections, 3 malarial infections, 1 HBV infection, 2 Staphylococcus aureus sepsis, 3 transfusion-related acute lung injuries, and 1 hemolytic transfusion reaction).
CONCLUSIONS
This is the first nationwide data regarding serious ATRs in Korea and could contribute to the implementation of an effective hemovigilance system.
MeSH Terms
Figure
Cited by 3 articles
-
ABO-Incompatible Transfusion Events Reported in Written Judgments and in the Korean Hemovigilance System
Sooin Choi, Jungwon Hyun, HongBi Yu, Duck Cho
Ann Lab Med. 2021;41(5):493-498. doi: 10.3343/alm.2021.41.5.493.Current State of Blood Management Services in Korea
Hyun Ok Kim
Ann Lab Med. 2022;42(3):306-313. doi: 10.3343/alm.2022.42.3.306.Preventing Blood Transfusion Errors: How Personal Digital Assistants Can Improve Patient Safety
Eunhui Ji, Kae Lyang Koo, Hyun Ki Min, Sun Min Lee, Seung-Hwan Oh, Hyun Ji Lee
Ann Lab Med. 2024;44(1):107-109. doi: 10.3343/alm.2023.44.1.107.
Reference
-
1. Cho JE. National blood management system and the direction of government policy in Korea. Korean J Hematol. 2010; 45:81–83. PMID: 21120182.
Article2. Kim S, Kim HO, Kim MJ, Lee SW, Shin YH, Choi YS, et al. Performance review of the National Blood Safety Improvement Project in Korea (2004-2009). Blood Res. 2013; 48:139–144. PMID: 23826584.
Article3. Andreu G, Morel P, Forestier F, Debeir J, Rebibo D, Janvier G, et al. Hemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion. 2002; 42:1356–1364. PMID: 12423521.
Article4. McClelland B, Love E, Scott S, Williamson LM. Haemovigilance: concept, Europe and UK initiatives. VoxSang. 1998; 74(Suppl 2):S431–S439.
Article5. Menitove JE. Hemovigilance in the United States of America. Vox Sang. 1998; 74(Suppl 2):S447–S455.
Article6. Keller-Stanislawski B, Lohmann A, Günay S, Heiden M, Funk MB. The German Haemovigilance System--reports of serious adverse transfusion reactions between 1997 and 2007. Transfus Med. 2009; 19:340–349. PMID: 19725904.
Article7. Otsubo H, Yamaguchi K. Current risks in blood transfusion in Japan. Jpn J Infect Dis. 2008; 61:427–433. PMID: 19050347.8. Faber JC. Review of the main haemovigilance systems in the world. Transfus Clin Biol. 2009; 16:86–92. PMID: 19442556.9. Joint United Kingdom Blood Transfusion and Tissue Transplantation Services (UKBTS) Professional Advisory Committee. Definition Look Back and Trace Back January 2015 Page 1 of 1. Updated on Jan 2015. http://www.transfusionguidelines.org.uk/document-library/general-documents.10. Kim MJ, Park Q, Min HK, Kim HO. Residual risk of transfusion-transmitted infection with human immunodeficiency virus, hepatitis C virus, and hepatitis B virus in Korea from 2000 through 2010. BMC Infect Dis. 2012; 12:160. PMID: 22817275.
Article11. Zou S, Stramer SL, Notari EP, Kuhns MC, Krysztof D, Musavi F, et al. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion. 2009; 49:1609–1620. PMID: 19413732.
Article12. Brant LJ, Reynolds C, Byrne L, Davison KL. Hepatitis B and residual risk of infection in English and Welsh blood donors, 1996 through 2008. Transfusion. 2011; 51:1493–1502. PMID: 21470235.
Article13. Moiz B, Moatter T, Shaikh U, Adil S, Ali N, Mahar F, et al. Estimating window period blood donations for human immunodeficiency virus Type 1, hepatitis C virus, and hepatitis B virus by nucleic acid amplification testing in Southern Pakistan. Transfusion. 2014; 54:1652–1659. PMID: 24383918.14. Feighner BH, Pak SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the republic of Korea. Emerg Infect Dis. 1998; 4:295–297. PMID: 9621202.
Article15. Seo DH, Cho YH, Yeo WH, Hwang BK, Jung HJ, Hwang YS, et al. Analysis of blood donation history of Korean malaria patients. Korean J Clin Pathol. 1999; 19:569–571.16. Korean Society of Blood Transfusion. The recommendation of standard blood bank and blood center service. 2nd ed. Seoul: Medrang Inforang;2007.17. Purdy E, Perry E, Gorlin J, Jensen K. Transfusion-transmitted malaria: unpreventable by current donor exclusion guidelines? Transfusion. 2004; 44:464. PMID: 14996210.
Article18. World Health Organization. World malaria report 2014. Geneva: WHO;2014.19. Cho HI, Seo DH, Kim HO. History of the Korean society of blood transfusion and blood services in Korea. Korean J Blood Transfus. 2012; 23:97–106.20. US Food and Drug Administration. Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year 2012. Washington DC: US Food and Drug Administration;2014.21. Eder AF, Kennedy JM, Dy BA, Notari EP, Weiss JW, Fang CT, et al. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004-2006). Transfusion. 2007; 47:1134–1142. PMID: 17581147.
Article22. Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006; 20:273–282. PMID: 17008165.
Article23. Fujii Y, Shibata Y, Miyata S, Inaba S, Asai T, Hoshi Y, et al. Consecutive national surveys of ABO-incompatible blood transfusion in Japan. Vox Sang. 2009; 97:240–246. PMID: 19476605.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidence of Adverse Transfusion Reactions from an Institutional Hemovigilance System: A Single Center Study
- Incidence of Adverse Reaction to Transfusion in Pediatric Patients
- Actual Incidence of Transfusion-Related Adverse Reactions Compared with Transfusion-Related Signs or Symptoms and by Each Blood Product
- Reporting System of Transfusion Adverse Reaction Using Electronic Medical Records Data
- Relief System for Adverse Drug Reactions in Korea