Bench-top Comparison of Physical Properties of 4 Commercially-Available Self-Expanding Intracranial Stents
- Affiliations
-
- 1Department of Radiology, Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. dhlee@amc.seoul.kr
- 2Department of Neurosurgery, Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Angiovention, Ilsan, Korea.
- KMID: 2371756
- DOI: http://doi.org/10.5469/neuroint.2017.12.1.31
Abstract
- PURPOSE
To better understand the performance of four commercially available neurovascular stents in intracranial aneurysm embolization, the stents were compared in terms of their basic morphological and mechanical properties.
MATERIALS AND METHODS
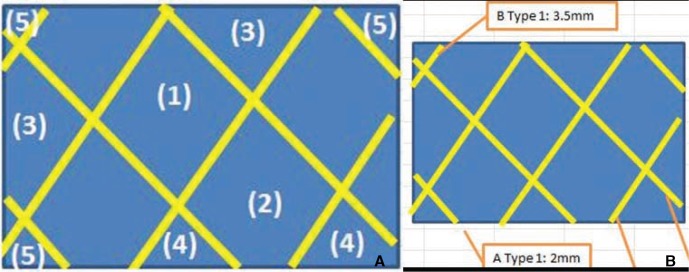
Four different types of stents that are currently being used for cerebral aneurysm embolization were prepared (two stents per type). Two were laser-cut stents (Neuroform and Enterprise) and two were braided from a single nitinol wire (LEO and LVIS stents). All were subjected to quantitative measurements of stent size, pore density, metal coverage, the force needed to load, push, and deploy the stent, radial force on deployment, surface roughness, and corrosion resistance.
RESULTS
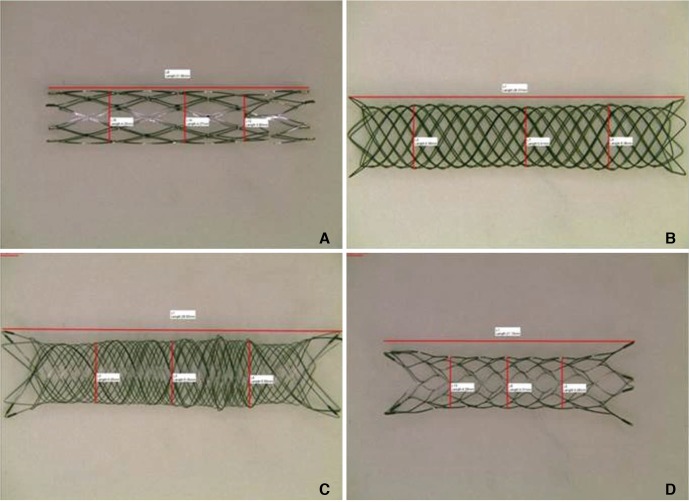
Compared to their nominal diameters, all stents had greater diameters after deployment. The length generally decreased after deployment. This was particularly marked in the braided stents. The braided stents also had higher pore densities than the laser-cut stents. Metal coverage was highest in the LEO stent (14%) and lowest in the Enterprise stent (5%). The LIVS stent had the highest microcatheter loading force (81.5 gf). The LEO stent had the highest passage force (55.0 gf) and deployment force (78.9 gf). The LVIS and LEO stents had the highest perpendicular (37.1 gf) and circumferential (178.4 gf) radial forces, respectively. The Enterprise stent had the roughest stent wire, followed by the LVIS, LEO, and Neuroform stents.
CONCLUSION
The four neurovascular stent types differed in terms of morphological and physical characteristics. An understanding of this diversity may help to decide which stent is most suitable for specific clinical situations.
MeSH Terms
Figure
Cited by 5 articles
-
Alpha Stent for Coiling of Unruptured, Wide-Necked, Distal Internal Carotid Artery Aneurysms: Safety and Effectiveness at 6 Months
Yunsun Song, Jae Jon Sheen, Joong-Goo Kim, Sang Hun Lee, Su Hee Cho, Jung Cheol Park, Choong Gon Choi, Deok Hee Lee
Korean J Radiol. 2020;21(2):228-235. doi: 10.3348/kjr.2019.0188.In Vitro Evaluation of Fusiform-Shaped Stents for Wide-Neck Intracranial Aneurysm Treatment
Zhen Yu Jia, Yuan Yuan Jiang, Jung Min Woo, Seon Moon Hwang, Ok Kyun Lim, Tae Il Kim, Jung Cheol Park, Hee Sun Lee, Eun Sang Kim, Deok Hee Lee
Neurointervention. 2018;13(2):117-123. doi: 10.5469/neuroint.2018.00976.Staged Approach for Stent-Assisted Coiling of Cerebral Aneurysms after Failure of Initial Intra-Saccular Catheterization
Abdulrahman Hamad Al-Abdulwahhab, Deok Hee Lee, Yunsun Song, Dae Chul Suh
Neurointervention. 2021;16(1):46-51. doi: 10.5469/neuroint.2020.00374.Overlapping Stents-Assisted Coiling for Vertebral Artery Dissecting Aneurysm : LVIS Stent within Neuroform EZ Stent
Xing-Long Liu, Bin Wang, Lin-Bo Zhao, Zhen-Yu Jia, Hai-Bin Shi, Sheng Liu
J Korean Neurosurg Soc. 2022;65(4):523-530. doi: 10.3340/jkns.2021.0275.Silent Embolic Infarction after Neuroform Atlas Stent-Assisted Coiling of Unruptured Intracranial Aneurysms
Seungho Shin, Lee Hwangbo, Tae-Hong Lee, Jun Kyeung Ko
J Korean Neurosurg Soc. 2024;67(1):42-49. doi: 10.3340/jkns.2023.0091.
Reference
-
1. Bing F, Darsaut TE, Salazkin I, Makoyeva A, Gevry G, Raymond J. Stents and flow diverters in the treatment of aneurysms: Device deformation in vivo may alter porosity and impact efficacy. Neuroradiology. 2013; 55:85–92. PMID: 22895818.
Article2. Gross BA, Frerichs KU. Stent usage in the treatment of intracranial aneurysms: past, present and future. J Neurol Neurosurg Psychiatry. 2013; 84:244–253. PMID: 23138770.
Article3. Izar B, Rai A, Raghuram K, Rotruck J, Carpenter J. Comparison of devices used for stent-assisted coiling of intracranial aneurysms. PloS one. 2011; 6:e24875. PMID: 21966374.
Article4. Peluso JP, van Rooij WJ, Sluzewski M, Beute GN. A new self-expandable nitinol stent for the treatment of wide-neck aneurysms: Initial clinical experience. AJNR Am J Neuroradiol. 2008; 29:1405–1408. PMID: 18436611.
Article5. Duda SH, Wiskirchen J, Tepe G, Bitzer M, Kaulich TW, Stoeckel D, et al. Physical properties of endovascular stents: An experimental comparison. J Vasc Interv Radiol. 2000; 11:645–654. PMID: 10834499.
Article6. Krischek O, Miloslavski E, Fischer S, Shrivastava S, Henkes H. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg. 2011; 54:21–28. PMID: 21506064.
Article7. Heller RS, Malek AM. Delivery technique plays an important role in determining vessel wall apposition of the Enterprise self-expanding intracranial stent. J Neurointerv Surg. 2011; 3:340–343. PMID: 21990459.
Article8. Kim BM, Kim DJ, Kim DI. Stent application for the treatment of cerebral aneurysms. Neurointervention. 2011; 6:53–70. PMID: 22125751.
Article9. Voute MT, Hendriks JM, van Laanen JHH, Pattynama PMT, Muhs BE, Poldermans D, et al. Radial force measurements in carotid stents: Influence of stent design and length of the lesion. J Vasc Interv Radiol. 2011; 22:661–666. PMID: 21514520.10. Müller-Hulsbeck S, Schäfer PJ, Charalambous N, Schaffner SR, Heller M, Jahnke T. Comparison of carotid stents: an in-vitro experiment focusing on stent design. J Endovasc Ther. 2009; 16:168–177. PMID: 19456191.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bench-Top Comparison of Three Different Types of Stents Used for Treatment of Intracranial Atherosclerotic Stenosis
- Stent Application for the Treatment of Cerebral Aneurysms
- Endovascular Treatment of Blood Blister-Like Aneurysms Using Multiple Self-Expanding Stents
- The challenges: Stent materials from the perspective of the manufacturer
- Modified 'Y-Configured Stents with Waffle Cone Technique' for Broad Neck Basilar Top Aneurysm