Allergy Asthma Immunol Res.
2017 May;9(3):281-284. 10.4168/aair.2017.9.3.281.
A Case of Cycloserine-Induced Lichenoid Drug Eruption Supported by the Lymphocyte Transformation Test
- Affiliations
-
- 1Department of Pediatrics, Kangwon National University School of Medicine, Chuncheon, Korea.
- 2The Research Department, Kangwon Regional Cancer Center, Kangwon National University Hospital, Chuncheon, Korea.
- 3Department of Internal Medicine, Kangwon National University Hospital, Chuncheon, Korea. legent@hanmail.net
- 4Department of Dermatology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 5Department of Allergy and Clinical Immunology, Kangwon National University School of Medicine, Chuncheon, Korea.
- KMID: 2371730
- DOI: http://doi.org/10.4168/aair.2017.9.3.281
Abstract
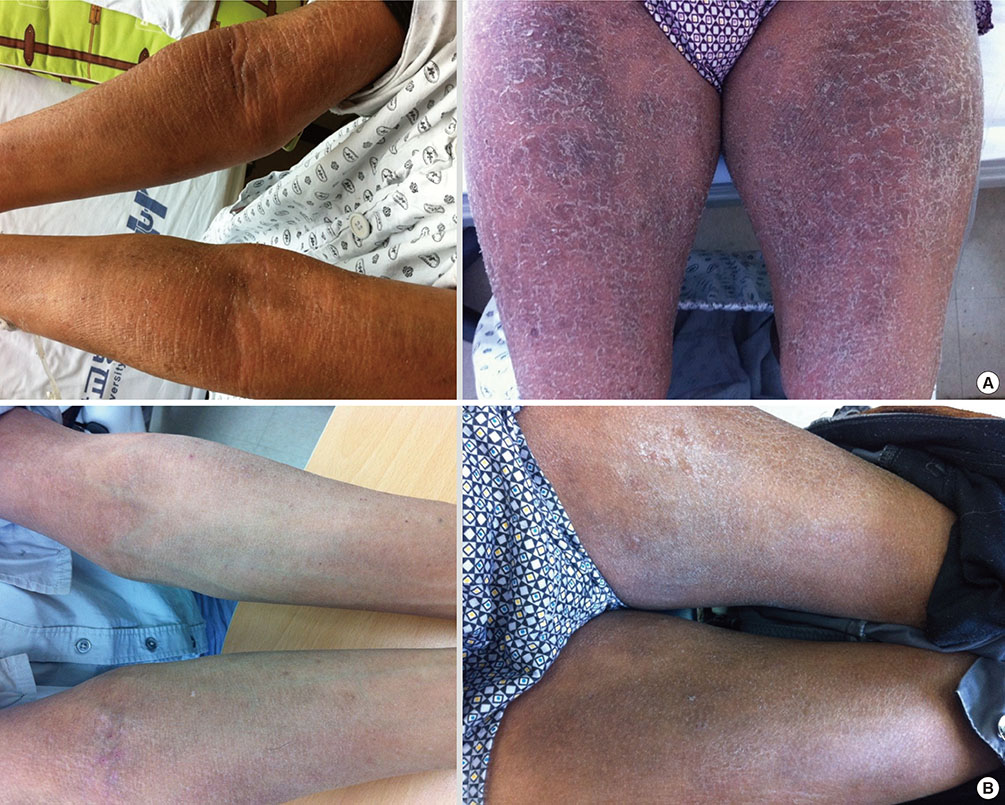
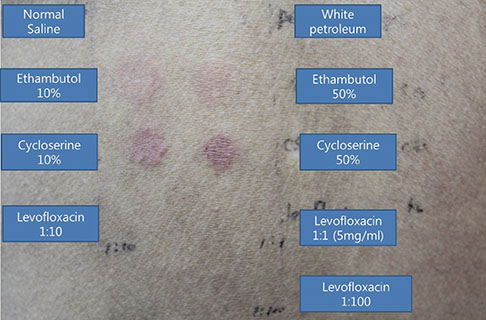
- Lichenoid drug eruption (LDE) is a rare form of delayed-type drug eruption. Among anti-tuberculosis (Tb) agents, cycloserine (CS) has been reported as a rare cause of LDE. Positive results on the lymphocyte transformation test (LTT) have not been reported in patients with LDE. In the present case, we performed LTT and a patch test, and successfully proved CS as the offending drug in this patient, who had been treated with multiple anti-Tb drugs. These observations suggest that CS should be considered a possible cause of LDE and that LTT can be an option for the diagnosis of LDE.
Keyword
MeSH Terms
Figure
Reference
-
1. Grossman ME, Warren K, Mady A, Satra KH. Lichenoid eruption associated with ethambutol. J Am Acad Dermatol. 1995; 33:675–676.2. Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. 1993; 29:249–255.3. Payette MJ, Weston G, Humphrey S, Yu J, Holland KE. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015; 33:631–643.4. Sugermann PB, Savage NW, Seymour GJ, Walsh LJ. Is there a role for tumor necrosis factor-alpha (TNF-alpha) in oral lichen planus? J Oral Pathol Med. 1996; 25:219–224.5. Kolm I, Eggmann N, Kamarashev J, Kerl K, French LE, Hofbauer GF. Lichenoid drug eruption following intravenous application of orally formulated diamorphine, a semisynthetic heroin. Case Rep Dermatol. 2013; 5:176–180.6. Frentz G, Wadskov S, Kassis V. Ethambutol-induced lichenoid eruption. Acta Derm Venereol. 1981; 61:89–91.7. Thakur BK, Verma S, Mishra J. Lichenoid drug reaction to isoniazid presenting as exfoliative dermatitis in a patient with acquired immunodeficiency syndrome. Int J STD AIDS. 2015; 26:512–515.8. Shim JH, Kim TY, Kim HO, Kim CW. Cycloserine-induced lichenoid drug eruption. Dermatology. 1995; 191:142–144.9. Shahul HA, Manu MK, Mohapatra AK, Mathew M. Rifampicin-induced lichenoid eruptions. BMJ Case Rep. 2014; 2014:bcr2013202346.10. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A;. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001; 45:321–328.11. Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010; 9:67–78.12. Tan WC, Ong CK, Kang SC, Razak MA. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007; 62:143–146.13. Na CH, Seo HD, Chung BS, Shin BS. A case of lichenoid drug eruption with whole body and oral mucosal involvement caused by antituberculosis drugs. Korean J Dermatol. 2008; 46:1145–1148.14. Choonhakarn C, Janma J. Pyrazinamide-induced lichenoid photodermatitis. J Am Acad Dermatol. 1999; 40:645–646.15. Suzuki Y, Miwa S, Shirai M, Ohba H, Murakami M, Fujita K, et al. Drug lymphocyte stimulation test in the diagnosis of adverse reactions to antituberculosis drugs. Chest. 2008; 134:1027–1032.16. Laine J, Happonen RP, Vainio O, Kalimo K. In vitro lymphocyte proliferation test in the diagnosis of oral mucosal hypersensitivity reactions to dental amalgam. J Oral Pathol Med. 1997; 26:362–366.17. Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996; 9:2026–2030.18. Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, et al. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci U S A. 2002; 99:3609–3614.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Allopurinol-Induced Fixed Drug Eruption Confirmed With a Lymphocyte Transformation Test
- A Case Report of Pirfenidone-Induced Lichenoid Drug Eruption in a Patient with Idiopathic Pulmonary Fibrosis
- A Case of Lichenoid Drug Eruption Associated with Imatinib Mesylate
- Lichenoid Drug Eruption Developed in Melanoma Patient Treated with Nivolumab
- Photosensitive Lichenoid Drug Eruption Due to Pembrolizumab in a Patient with Non-Small Cell Lung Cancer