Korean J Ophthalmol.
2015 Oct;29(5):325-330. 10.3341/kjo.2015.29.5.325.
Sterile Inflammation after Intravitreal Injection of Aflibercept in a Korean Population
- Affiliations
-
- 1Nune Eye Hospital, Seoul, Korea. kim1441@gmail.com
- KMID: 2363806
- DOI: http://doi.org/10.3341/kjo.2015.29.5.325
Abstract
- PURPOSE
To report the frequency and clinical features of sterile inflammation after intravitreal aflibercept injection in a Korean population.
METHODS
A single-center, retrospective study was performed in patients who received intravitreal aflibercept from July 2013 through January 2015.
RESULTS
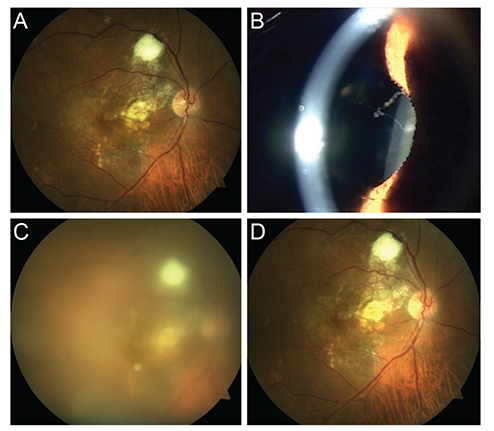
A total of four cases of post-injection sterile inflammation were identified from 723 aflibercept injections in 233 patients. Patients presented 1 to 13 days after intravitreal aflibercept injection (mean, 5 days). The mean baseline visual acuity was 20 / 60, which decreased to 20 / 112 at diagnosis but ultimately recovered to 20 / 60. Three cases had inflammatory cells in the anterior chamber (mean, 2.25+; range, 0 to 4+), and all cases had vitritis (mean, 3+; range, 2+ to 4+). No patients had pain. Only one patient underwent anterior chamber sampling (culture negative) and injection of antibiotics. Three of four patients were treated with a topical steroid, and all experienced improvement in their symptoms and signs of inflammation.
CONCLUSIONS
The overall incidence of sterile inflammation after intravitreal aflibercept injection in a Korean population was 4 of 723 injections (0.55%), or 4 of 233 patients (1.79%). Sterile inflammation after intravitreal aflibercept injection typically presents without pain, and the visual outcomes are generally favorable.
MeSH Terms
-
Aged
Aged, 80 and over
Female
Follow-Up Studies
Humans
Incidence
Intravitreal Injections
Macular Edema/*drug therapy/epidemiology
Male
Middle Aged
Receptors, Vascular Endothelial Growth Factor/*administration & dosage
Recombinant Fusion Proteins/*administration & dosage
Republic of Korea/epidemiology
Retrospective Studies
Treatment Outcome
Vascular Endothelial Growth Factor A
Visual Acuity
Receptors, Vascular Endothelial Growth Factor
Recombinant Fusion Proteins
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.3. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1124–1133.e1.4. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1102–1112.e1.5. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.6. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012; 119:802–809.7. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013; 120:2013–2022.8. Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014; 121:1045–1053.9. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–2548.10. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014; 121:2247–2254.11. Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO Study. Ophthalmology. 2014; 121:202–208.12. Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal af libercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014; 121:193–201.13. Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015; 122:538–544.14. Hahn P, Kim JE, Stinnett S, et al. Aflibercept-related sterile inflammation. Ophthalmology. 2013; 120:1100–1101.e1-5.15. Fine HF, Roth DB, Shah SP, et al. Frequency and characteristics of intraocular inflammation after aflibercept injection. Retina. 2015; 35:681–686.16. Goldberg RA, Shah CP, Wiegand TW, Heier JS. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol. 2014; 158:733–737.17. Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:779–781.18. Fauser S, Schroeder S, Caramoy A, et al. Intraocular inflammation after intravitreal ranibizumab injections. Acta Ophthalmol. 2011; 89:e98–e99.19. Kay CN, Tarantola RM, Gehrs KM, et al. Uveitis following intravitreal bevacizumab: a non-infectious cluster. Ophthalmic Surg Lasers Imaging. 2011; 42:292–296.20. Wickremasinghe SS, Michalova K, Gilhotra J, et al. Acute intraocular inflammation after intravitreous injections of bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology. 2008; 115:1911–1915.21. Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol. 2008; 246:81–87.22. Georgopoulos M, Polak K, Prager F, et al. Characteristics of severe intraocular inflammation following intravitreal injection of bevacizumab (Avastin). Br J Ophthalmol. 2009; 93:457–462.23. Antoszyk AN, Tuomi L, Chung CY, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008; 145:862–874.24. Marticorena J, Romano V, Gomez-Ulla F. Sterile endophthalmitis after intravitreal injections. Mediators Inflamm. 2012; 2012:928123.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Noninfectious Endophthalmitis after Intravitreal Injection of Aflibercept
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- Haller Layer Thickness after Intravitreal Aflibercept Injection in Diabetic Macular Edema: 1 Month Change
- A Case of Nonarteritic Anterior Ischemic Optic Neuropathy after Intravitreal Aflibercept Injection
- Clinical Manifestations of Presumed Sterile Endophthalmitis After Intravitreal Triamcinolone Injection