J Korean Med Sci.
2016 Feb;31(Suppl 1):S59-S68. 10.3346/jkms.2016.31.S1.S59.
Radiological Justification for and Optimization of Nuclear Medicine Practices in Korea
- Affiliations
-
- 1Department of Nuclear Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea. kimbi@kirams.re.kr
- KMID: 2363382
- DOI: http://doi.org/10.3346/jkms.2016.31.S1.S59
Abstract
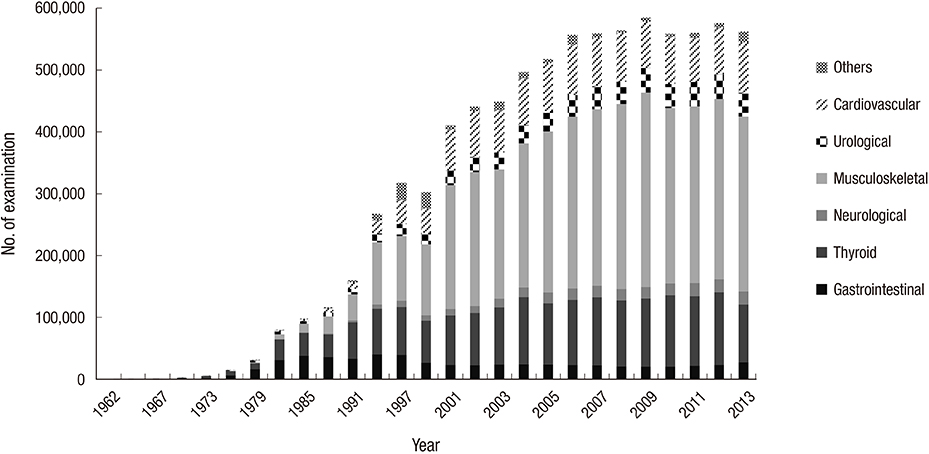
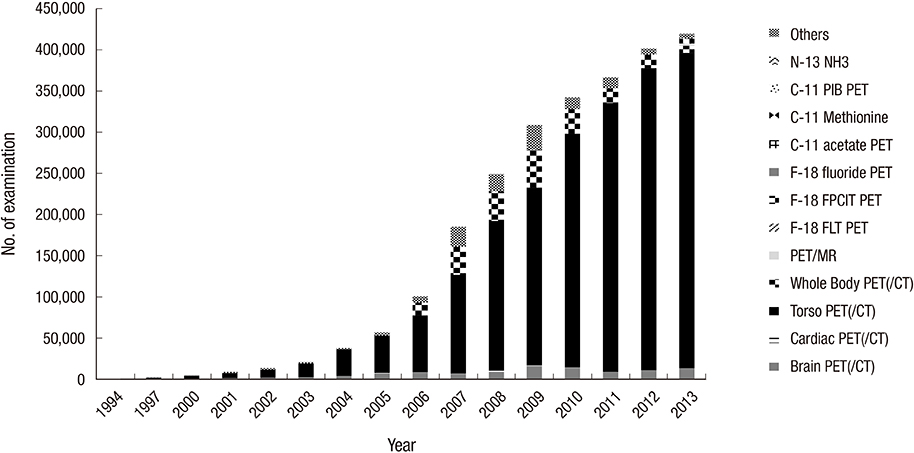
- Nuclear medicine is a rapidly growing discipline that employs advanced novel hybrid techniques that provide unique anatomical and functional information, as well as targets for molecular therapy. Concomitantly, there has been an increase in the attention paid to medical radiation exposure. A radiological justification for the practice of nuclear medicine has been implemented mainly through referral guidelines based on research results such as prospective randomized clinical trials. The International Commission on Radiological Protection recommends diagnostic reference levels as a practical mechanism to optimize medical radiation exposure in order to be commensurate with the medical purpose. The Korean Society of Nuclear Medicine has been implementing radiological optimization through a survey of the protocols on how each hospital determines the dose of administration of each radiopharmaceutical. In the case of nuclear medicine, radiation exposure of caregivers and comforters of patients discharged after administration of therapeutic radiopharmaceuticals can occur; therefore, optimization has been implemented through written instructions for patients, based on international recommendations. The development of patient-radiation-dose monitoring software, and a national registry and management system of patient-radiation-dose is needed to implement radiological optimization through diagnostic reference levels. This management system must work in agreement with the "Institute for Quality Management of Nuclear Medicine", and must take into account the medical reality of Korea, such as low medicine fee, in order to implement reasonable radiological justification and optimization.
MeSH Terms
Figure
Reference
-
1. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007; 37:1–332.2. The Society of Nuclear Medicine and Molecular Imaging. 18F-fluorodeoxyglucose (FDG) PET and PET/CT practice guidelines in oncology: a summary of the recommendations and practice guidelines of professional groups. accessed on 3 October 2015. Available at http://interactive.snm.org/docs/PET_PROS/OncologyPracticeGuidelineSummary.pdf.3. The Society of Nuclear Medicine and Molecular Imaging. Part I: NCCN practice guidelines tabular summary (DD 3/20/13): PET and PET/CT. accessed on 3 October 2015. Available at http://snmmi.files.cms-plus.com/FileDownloads/Centers/NCCNPracticeGuidelinesI.pdf.4. The Society of Nuclear Medicine and Molecular Imaging. Part II: NCCN practice guidelines narrative summary: PET and PET/CT. accessed on 3 October 2015. Available at http://snmmi.files.cms-plus.com/FileDownloads/Centers/NCCNPracticeGuidelinesII.pdf.5. The Society of Nuclear Medicine and Molecular Imaging. Part III: NCCN practice guidelines: focus areas for F 18 fluorodeoxyglucose (FDG) PET/CT evidence development (4.3.13). accessed on 3 October 2015. Available at http://snmmi.files.cms-plus.com/docs/PET_PROS/NCCNPracticeGuidelinesIII.pdf.6. Podoloff DA, Ball DW, Ben-Josef E, Benson AB 3rd, Cohen SJ, Coleman RE, Delbeke D, Ho M, Ilson DH, Kalemkerian GP, et al. NCCN task force: clinical utility of PET in a variety of tumor types. J Natl Compr Canc Netw. 2009; 7:Suppl 2. S1–26.7. The Society of Nuclear Medicine and Molecular Imaging. Cardiac PET and PET/CT imaging practice guidelines: a summary of the recommendations and practice guidelines of professional groups. accessed on 3 October 2015. Available at http://www.snm.org/docs/PET_PROS/CardiacPracticeGuidelinesSummary.pdf.8. The American College of Radiology. ACR's appropriateness criteria®. accessed on 3 October 2015. Available at http://www.acr.org/Quality-Safety/Appropriateness-Criteria.9. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.10. Kim BI. Clinical application of F-18 FDG PET (PET/CT) in colo-rectal and anal cancer. Nucl Med Mol Imaging. 2008; 42:52–59.11. Yi KH, Park YJ, Koong SS, Kim JH, Na DG, Ryu JS, Park SY, Park IA, Baek CH, Shong YK, et al. Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Korean J Otorhinolaryngol-Head Neck Surg. 2011; 54:8–36.12. Kim WB, Kim TY, Kwon HS, Moon WJ, Lee JB, Choi YS, Kim SK, Kim SW, Chung KW, Baek JH, et al. Management guidelines for patients with thyroid nodules and thyroid cancer. J Korean Endocr Soc. 2007; 22:157–187.13. The International Commission on Radiological Protection. ICRP Publication 105. Radiation protection in medicine. Ann ICRP. 2007; 37:1–63.14. The American College of Radiology and the American Association of Physicists in Medicine. ACR-AAPM practice parameter for reference levels and achievable administered activity for nuclear medicine and molecular imaging. 2015 (Resolution 53). accessed on 3 October 2015. Available at http://www.acr.org/Quality-Safety/Standards-Guidelines/Practice-Guidelines-by-Modality/Nuclear-Medicine.15. The Society of Nuclear Medicine and Molecular Imaging. SNMMI Procedure Standard 2015. accessed on 3 October 2015. Available at http://www.snmmi.org/ClinicalPractice/content.aspx?ItemNumber=6414&navItemNumber=10790.16. The Society of Nuclear Medicine and Molecular Imaging Dose Optimization Task Force. Nuclear medicine radiation dose tool 2015. accessed on 3 October 2015. Available at http://www.snmmi.org/ClinicalPractice/doseTool.aspx?ItemNumber=11216&navItemNumber=11218.17. Lassmann M, Treves ST; EANM/SNMMI Paediatric Dosage Harmonization Working Group. Paediatric radiopharmaceutical administration: harmonization of the 2007 EANM paediatric dosage card (version 1.5.2008) and the 2010 North American consensus guidelines. Eur J Nucl Med Mol Imaging. 2014; 41:1036–1041.18. The Intersocietal Accreditation Commission. The IAC standards and guidelines for Nuclear/PET accreditation. accessed on 8 October 2015. Available at http://www.intersocietal.org/nuclear/standards/IACNuclearPETStandards2012.pdf.19. Rehani MM. Dose surveys and DRLs: critical look and way forward. Radiat Prot Dosimetry. 2015; 165:67–69.20. The Korean Society of Nuclear Medicine. KSNM nuclear medicine procedure manual. accessed on 9 October 2015. Available at http://www.ksnm.or.kr/upload/education/201.hwp.21. The Korean Society of Nuclear Medicine. F-18 FDG PET/CT procedure standard. 2015. accessed on 9 October 2015. Available at http://www.ksnm.or.kr/upload/education/201.hwp.22. The International Commission on Radiological Protection. Release of patients after therapy with unsealed radionuclides. ICRP Publication 94. Ann ICRP. 2004; 34:1–80.23. The International Commission on Radiological Protection. Radiological Protection and Safety in Medicine. ICRP Publication 73. Ann ICRP. 1996; 26:1–47.24. The International Atomic Energy Agency. Patient instruction card. accessed on 15 October 2015. Available at https://rpop.iaea.org/RPOP/RPoP/Content/Documents/Whitepapers/patient-information.pdf.25. American Thyroid Association Taskforce On Radioiodine Safety. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid. 2011; 21:335–346.26. The Medical Radiation Safety Research Center. Radiation safety instruction for the patients with thyroid cancer after I-131 therapy. accessed on 9 October 2015. Available at http://www.ksnmt.or.kr/bbs/skin/notice_popup2/download.php?code=notice&number=1304.27. Ryu YH. Clinical application of 18F-FDG PET in Alzheimer’s disease. Nucl Med Mol Imaging. 2008; 42:166–171.28. The Korean Society of Nuclear Medicine. KSNM technical standard for procedures using radiopharmaceuticals. accessed on 15 October 2015. Available at http://www.ksnm.or.kr/bbs/index.html?code=guan&category=&gubun=&page=1&number=695&mode=view&order=&sort=&keyfield=&key=&page_type=.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiation Safety in Nuclear Medicine Procedures
- CT radiation dose and radiation reduction strategies
- Strategies of computed tomography radiation dose reduction: justification and optimization
- Suggestion for Improper Radiologic Examination Using Ionizing Radiation
- General Principles of Radiation Protection in Fields of Diagnostic Medical Exposure