J Korean Med Sci.
2016 Jan;31(1):13-17. 10.3346/jkms.2016.31.1.13.
Immunogenicity and Safety of a Live Attenuated Zoster Vaccine (ZOSTAVAX(TM)) in Korean Adults
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. heejinmd@korea.ac.kr
- 2Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- 5Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2359986
- DOI: http://doi.org/10.3346/jkms.2016.31.1.13
Abstract
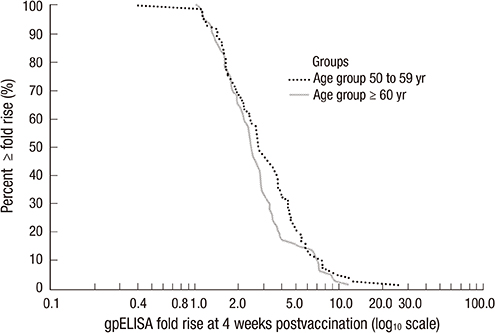
- A live attenuated zoster vaccine (ZOSTAVAX(TM), Merck & Co., Inc.) was approved by the Korea Ministry of Food and Drug Safety in 2009. However, the immunogenicity and safety of the vaccine has not been assessed in Korean population. This is multi-center, open-label, single-arm study performed with 180 healthy Korean adults > or =50 yr of age. The geometric mean titer (GMT) and geometric mean fold rise (GMFR) of varicella zoster virus (VZV) antibodies were measured by glycoprotein enzyme-linked immunosorbent assay (gpELISA) at 4 weeks post-vaccination. Subjects were followed for exposure to varicella or herpes zoster (HZ), the development of any varicella/varicella-like or HZ/HZ-like rashes, and any other clinical adverse experiences (AEs) for 42 days post-vaccination. For the 166 subjects included in the per-protocol population, the GMT at Day 1 was 66.9. At 4 weeks post-vaccination, the GMT for this population was 185.4, with a GMFR of 2.8 (95% CI, 2.5-3.1). Of the 180 subjects vaccinated, 62.8% experienced > or =1 AE, with 53.3% of subjects reporting injection-site AEs. The most frequently reported injection-site AEs were erythema (45.0%) with the majority being mild in intensity. Overall, 44 (24.4%) subjects experienced > or =1 systemic AE, 10 (5.5%) subjects experienced a systemic vaccine-related AE, and 3 (1.7%) subjects experienced > or =1 serious AE not related to vaccine. No subjects reported a VZV-like rash. There was no subject of death and no subject discontinued due to an adverse event. A single dose of zoster vaccine induced VZV-specific gpELISA antibody response and was generally well-tolerated in healthy Korean adults > or =50 yr of age (registry at www.clinicaltrial.gov No. NCT01556451).
MeSH Terms
Figure
Reference
-
1. Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004; 39:342–348.2. Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013; 369:255–263.3. Reid JS, Ah Wong B. Herpes zoster (shingles) at a large New Zealand general practice: incidence over 5 years. N Z Med J. 2014; 127:56–60.4. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014; 4:e004833.5. Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015; 70:178–186.6. Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013; 13:170.7. Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997-2002. Epidemiol Infect. 2005; 133:245–253.8. Choi WS, Noh JY, Huh JY, Jo YM, Lee J, Song JY, Kim WJ, Cheong HJ. Disease burden of herpes zoster in Korea. J Clin Virol. 2010; 47:325–329.9. Kim YJ, Lee CN, Lim CY, Jeon WS, Park YM. Population-based study of the epidemiology of herpes zoster in Korea. J Korean Med Sci. 2014; 29:1706–1710.10. Mick G, Gallais JL, Simon F, Pinchinat S, Bloch K, Beillat M, Serradell L, Derrough T. Burden of herpes zoster and postherpetic neuralgia: Incidence, proportion, and associated costs in the French population aged 50 or over. Rev Epidemiol Sante Publique. 2010; 58:393–401.11. Lin YH, Huang LM, Chang IS, Tsai FY, Lu CY, Shao PL, Chang LY. Varicella-Zoster Working Group. Advisory Committee on Immunization Practices, Taiwan. Disease burden and epidemiology of herpes zoster in pre-vaccine Taiwan. Vaccine. 2010; 28:1217–1220.12. Levi M, Bellini I, Capecchi L, Pieri L, Bechini A, Boccalini S, Callaioli S, Gasparini R, Panatto D, Tiscione E, et al. The burden of disease of Herpes Zoster in Tuscany. Hum Vaccin Immunother. 2015; 11:185–191.13. Song H, Lee J, Lee M, Choi WS, Choi JH, Lee MS, Hashemi M, Rampakakis E, Kawai K, White R, et al. Burden of illness, quality of life, and healthcare utilization among patients with herpes zoster in South Korea: a prospective clinical-epidemiological study. Int J Infect Dis. 2014; 20:23–30.14. Choi WS, Kwon SS, Lee J, Choi SM, Lee JS, Eom JS, Sohn JW, Choeng HJ. Immunity and the burden of herpes zoster. J Med Virol. 2014; 86:525–530.15. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005; 352:2271–2284.16. Schmader KE, Levin MJ, Gnann JW Jr, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012; 54:922–928.17. Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006; 78:1679–1687.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant zoster vaccine (Shingrix®): a new option for the prevention of herpes zoster and postherpetic neuralgia
- What is Different about Recombinant Herpes Zoster Vaccine?
- Optimal Timing of Zoster Vaccination After Shingles: A Prospective Study of the Immunogenicity and Safety of Live Zoster Vaccine
- Evaluation Methods for the Immunogenicity of Varicella and Zoster Vaccines
- Tow-Year Follow-up Study for Clinical Feature and Immunity of The Children, Vaccinated by 47 Passaged Oka Strain Live Attenuated Varicella Vaccine