J Korean Ophthalmol Soc.
2016 Oct;57(10):1619-1624. 10.3341/jkos.2016.57.10.1619.
The Efficacy of Brinzolamide 1%/Brimonidine 0.2% Fixed Combination in Normal Tension Glaucoma
- Affiliations
-
- 1Department of Ophthalmology, Dong-A University College of Medicine, Busan, Korea. jinnham@hanmail.net
- KMID: 2355419
- DOI: http://doi.org/10.3341/jkos.2016.57.10.1619
Abstract
- PURPOSE
To evaluate the efficacy and safety of brinzolamide 1%/brimonidine 0.2% fixed combination (BBFC) in normal tension glaucoma (NTG) patients.
METHODS
This prospective study included patients treated with brinzolamide 1% monotherapy, brimonidine 0.2% monotherapy or brinzolamide 1% and brimonidine 0.2% concomitant therapy, as well as newly diagnosed NTG patients. The enrolled patients who used brinzolamide 1% or brimonidine 0.2% switched to BBFC and newly diagnosed NTG patients were treated with BBFC. The patients receiving brinzolamide 1% or brimonidine 0.2% monotherapy or brinzolamide 1% and brimonidine 0.2% concomitant therapy switched antiglaucoma drugs to BBFC. Newly diagnosed NTG patients used BBFC as the first therapy. The study consisted of 1 screening/baseline visit and 3 follow-up visits conducted after 1, 4, 8, 12 and 24 weeks of treatment. Intraocular pressure (IOP), mean deviation value and adverse drug reactions were evaluated before treatment and after treatment with BBFC.
RESULTS
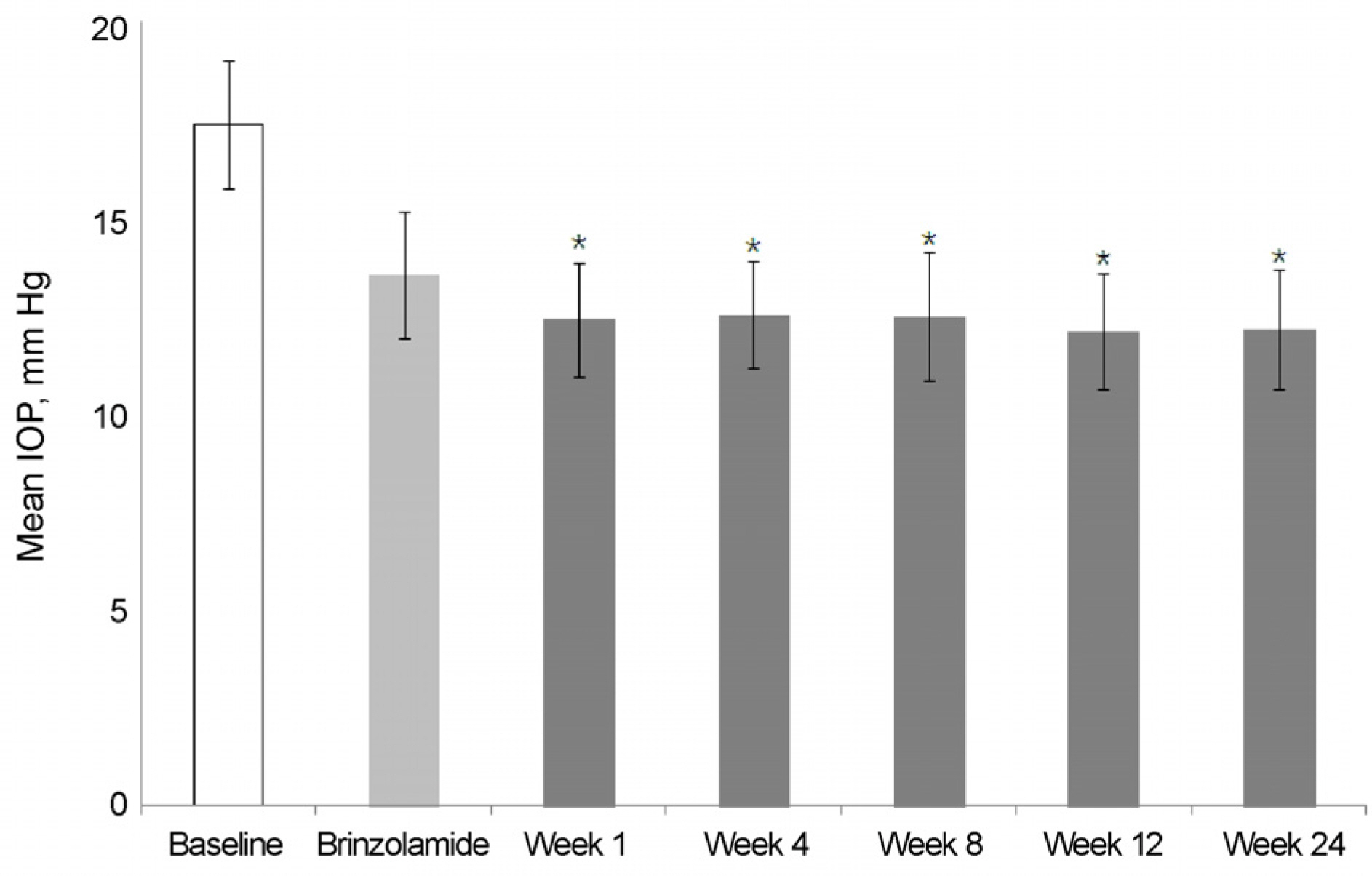
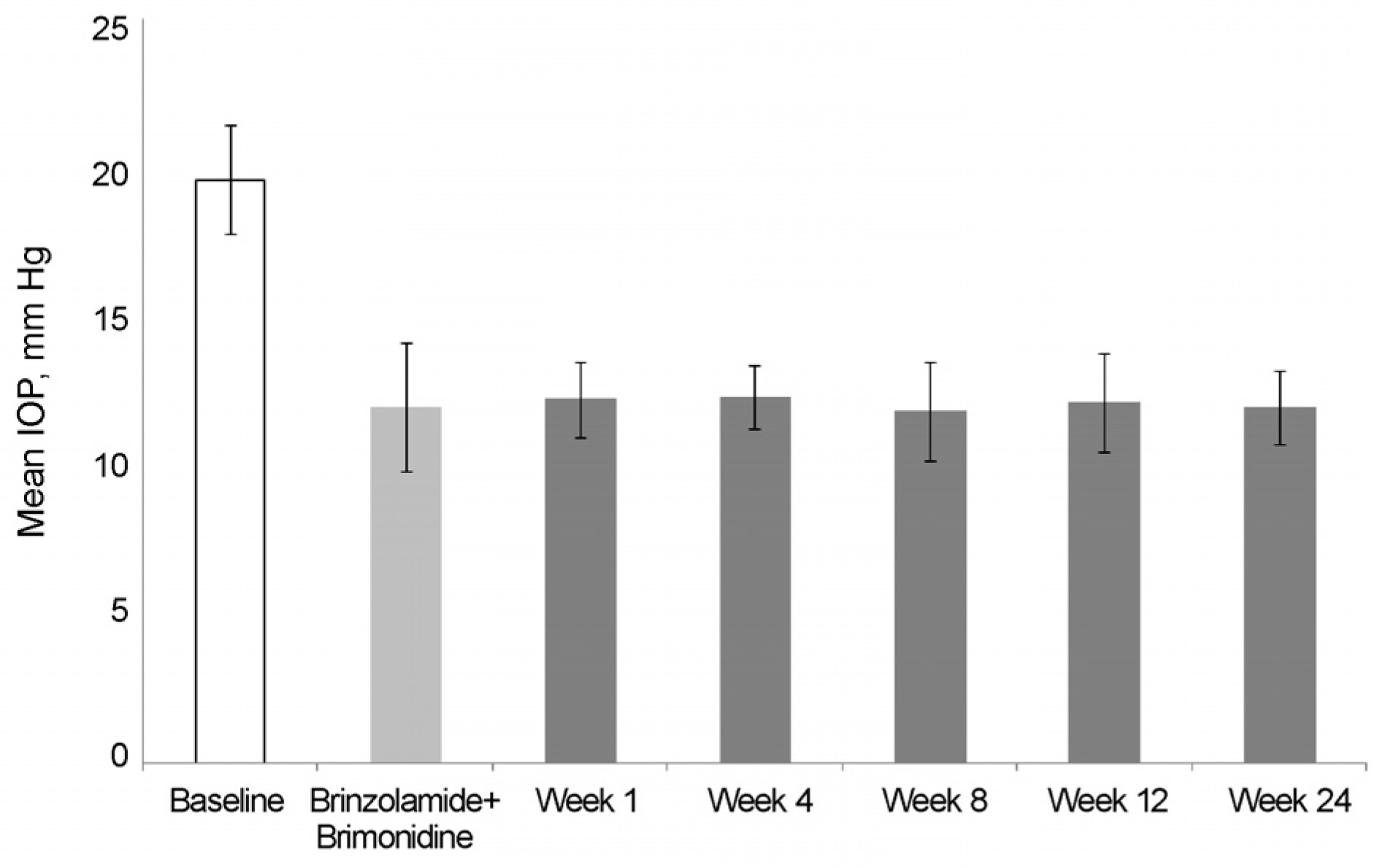
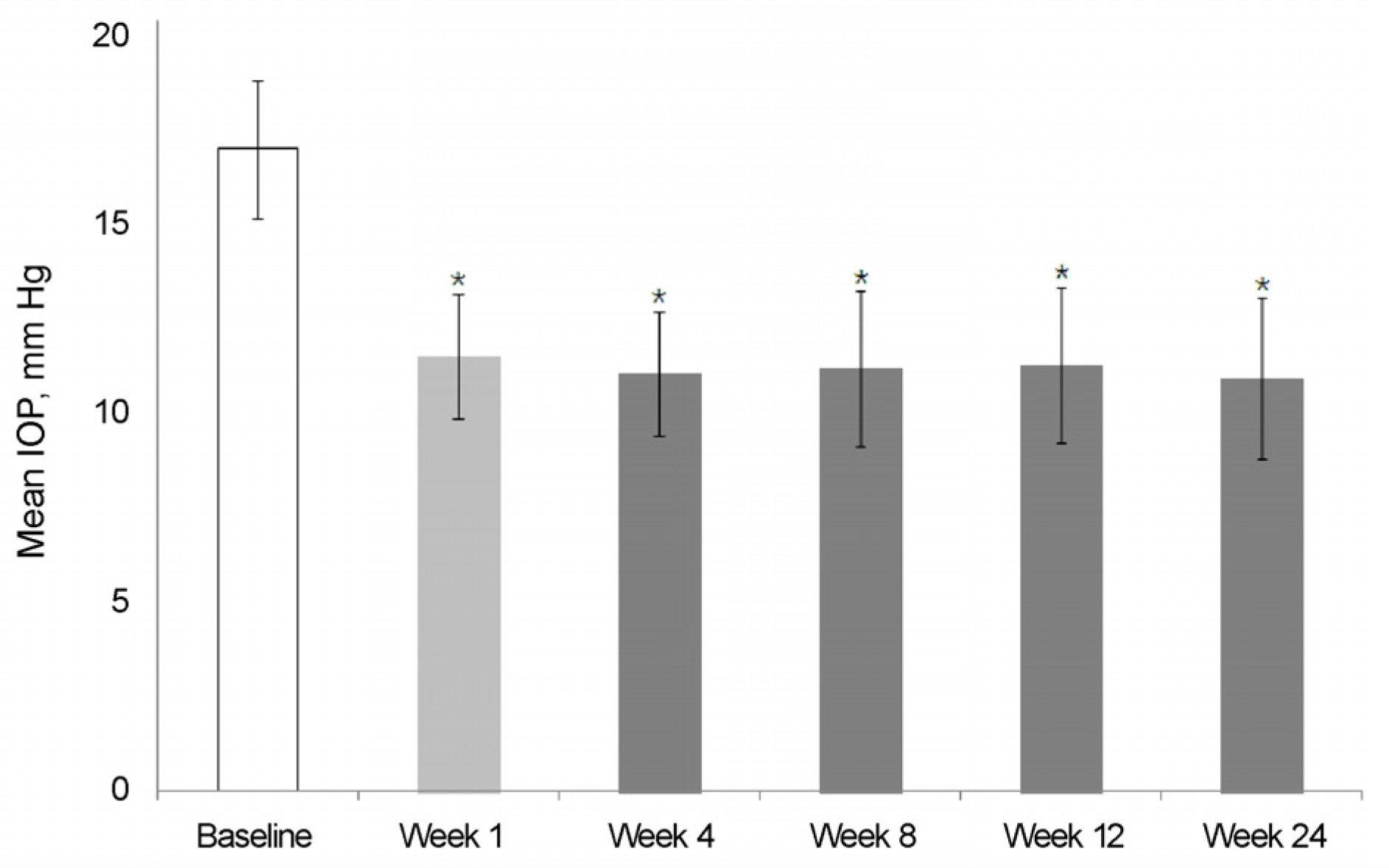
The mean IOP in the brinzolamide 1% monotherapy group was 13.5 ± 1.6 mm Hg and the mean IOP after switched from brinzolamide 1% monotherapy to BBFC was 12.1 ± 1.5 mm Hg. The mean IOP in the brimonidine 0.2% monotherapy group was 14.2 ± 1.3 mm Hg and the mean IOP after switched from brimonidine 0.2% monotherapy to BBFC was 11.7 ± 1.5 mm Hg. The mean IOP was 11.9 ± 2.1 mm Hg in the brinzolamide 1% and brimonidine 0.2% concomitant therapy group and the mean IOP after switched from brinzolamide 1% and brimonidine 0.2% concomitant therapy to BBFC was 12.0 ± 1.1 mm Hg. The mean IOP and reduction rate were 10.7 ± 2.1 mm Hg and 35.5%, respectively,in the newly diagnosed NTG patients treated with BBFC. There was no serious adverse drug reaction causing ocular damage.
CONCLUSIONS
BBFC provides a significant IOP reduction and is a safe antiglaucoma medication for NTG patients.
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Effectiveness of a Brinzolamide 1%-Brimonidine 0.2% Fixed Combination for Normal Tension Glaucoma in South Koreans
In Tae Kim, Jeong Hun Bae, Joon Mo Kim
J Korean Ophthalmol Soc. 2018;59(6):561-568. doi: 10.3341/jkos.2018.59.6.561.
Reference
-
References
1. Kamal D, Hitchings R. Normal tension glaucoma-a practical approach. Br J Ophthalmol. 1998; 82:835–40.
Article2. Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004; 111:1641–8.3. Kim CS, Seong GJ, Lee NH, et al. Prevalence of primary open-abdominal glaucoma in central South Korea the Namil study. Ophthalmology. 2011; 118:1024–30.4. Webers CA, Beckers HJ, Nuijts RM, Schouten JS. Pharmacological management of primary open-angle glaucoma: second-line options and beyond. Drugs Aging. 2008; 25:729–59.5. Quigley HA, Enger C, Katz J, et al. Risk factors for the abdominal of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994; 112:644–9.6. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701–13. 829–30.7. Barnebey HS, Orengo-Nania S, Flowers BE, et al. The safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol. 2005; 140:1–7.
Article8. Lee AJ, McCluskey P. Fixed combination of topical brimonidine 0.2% and timolol 0.5% for glaucoma and uncontrolled intraocular pressure. Clin Ophthalmol. 2008; 2:545–55.
Article9. Inoue K, Shiokawa M, Sugahara M, et al. Three-month evaluation of dorzolamide hydrochloride/timolol maleate fixed-combination eye drops versus the separate use of both drugs. Jpn J Ophthalmol. 2012; 56:559–63.
Article10. Higginbotham EJ, Hansen J, Davis EJ, et al. Glaucoma medication persistence with a fixed combination versus multiple bottles. Curr Med Res Opin. 2009; 25:2543–7.
Article11. Taniguchi T, Kitazawa Y. The potential systemic effect of topically applied beta-blockers in glaucoma therapy. Curr Opin Ophthalmol. 1997; 8:55–8.12. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998; 126:498–505.13. Wolfs RC, Borger PH, Ramrattan RS, et al. Changing views on open-angle glaucoma: definitions and prevalences-The Rotterdam Study. Invest Ophthalmol Vis Sci. 2000; 41:3309–21.14. Katz G, Dubiner H, Samples J, et al. Three-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%. JAMA Ophthalmol. 2013; 131:724–30.
Article15. Aung T, Laganovska G, Hernandez Paredes TJ, et al. Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension. Ophthalmology. 2014; 121:2348–55.
Article16. Gandolfi SA, Lim J, Sanseau AC, et al. Randomized trial of abdominal/brimonidine versus brinzolamide plus brimonidine for open-angle glaucoma or ocular hypertension. Adv Ther. 2014; 31:1213–27.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy and Safety of Preservative-containing and Preservative-free Brimonidine-Timolol Fixed Combination in Normal Tension Glaucoma
- Clinical Effectiveness of a Brinzolamide 1%-Brimonidine 0.2% Fixed Combination for Normal Tension Glaucoma in South Koreans
- Clinical Characteristics of Allergy to a Brinzolamide 1.0%/Brimonidine 0.2% Fixed Combination in Korean Glaucoma Patients
- Two Cases of Atypical Allergic Conjunctivitis Caused by Topical Administration of Brimonidine
- The Effect of Fixed Combination of Brinzolamide 1% and Timolol 0.5% in Normal-Tension Glaucoma