J Breast Cancer.
2014 Mar;17(1):91-97.
Concurrent Invasive Ductal Carcinoma of the Breast and Malignant Follicular Lymphoma, Initially Suspected to Be Metastatic Breast Cancer: A Case Report
- Affiliations
-
- 1Department of Radiology, Kyungpook National University Hospital, Daegu, Korea.
- 2Department of Radiology, Kyungpook National University Medical Center, Daegu, Korea. mamrad@knu.ac.kr
- 3Department of Pathology, Kyungpook National University Medical Center, Daegu, Korea.
- 4Department of Surgery, Kyungpook National University Medical Center, Daegu, Korea.
Abstract
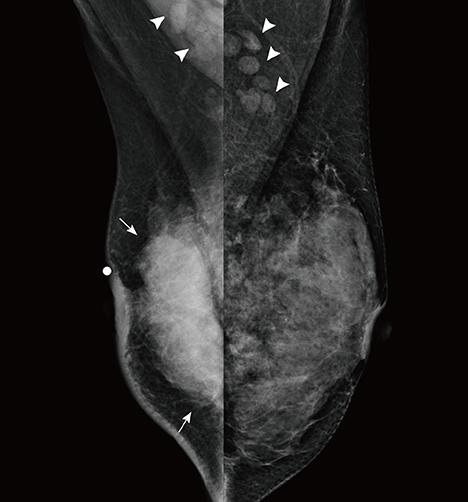
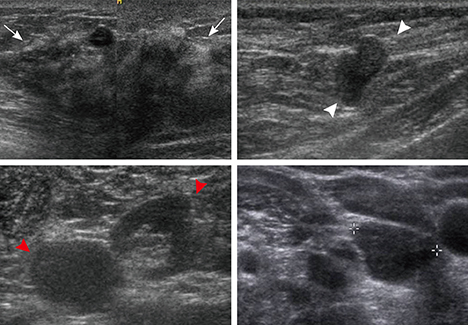
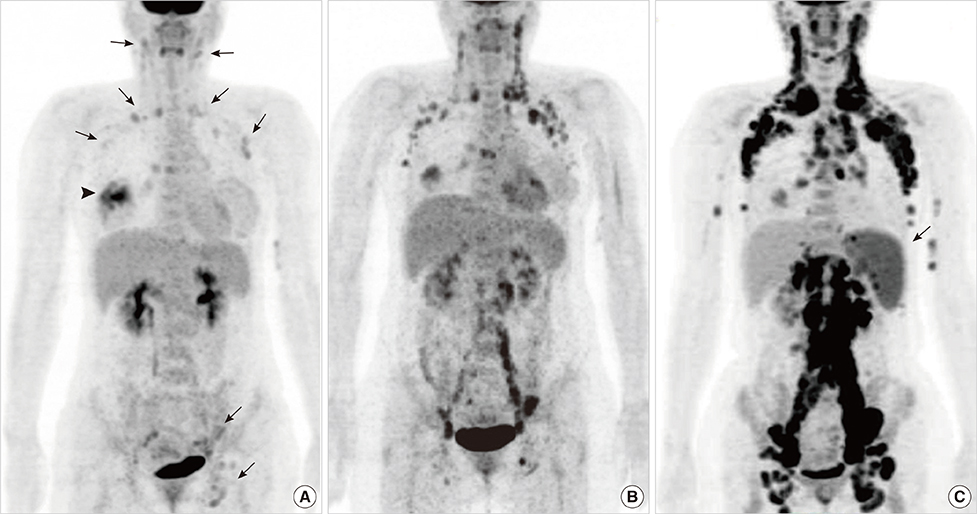
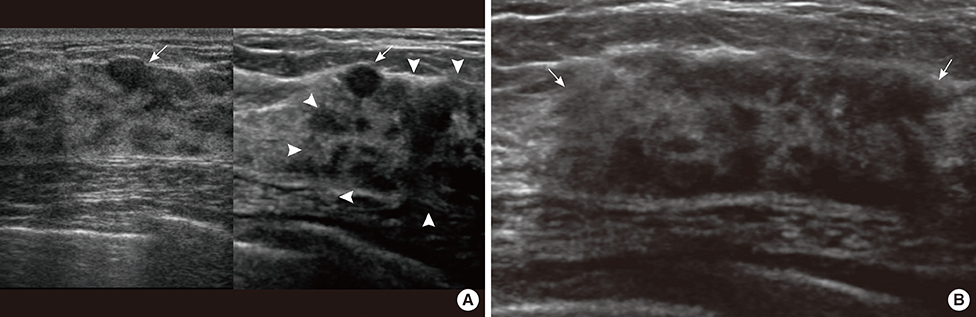
- This report describes a case of a 40-year-old female patient with concurrent invasive ductal carcinoma of the breast and malignant follicular lymphoma, initially suspected to be metastatic breast cancer. During the initial evaluation of invasive ductal carcinoma of right breast, multiple lymphadenopathies were noted throughout the body on ultrasonography and positron emission tomography/computed tomography images. Clinically, metastatic breast cancer was suggested, and the patient was administered chemotherapy, including hormonal therapy. The breast cancer improved slightly, but the lymphadenopathies progressed and excisional biopsy of a cervical lymph node revealed malignant follicular lymphoma.
MeSH Terms
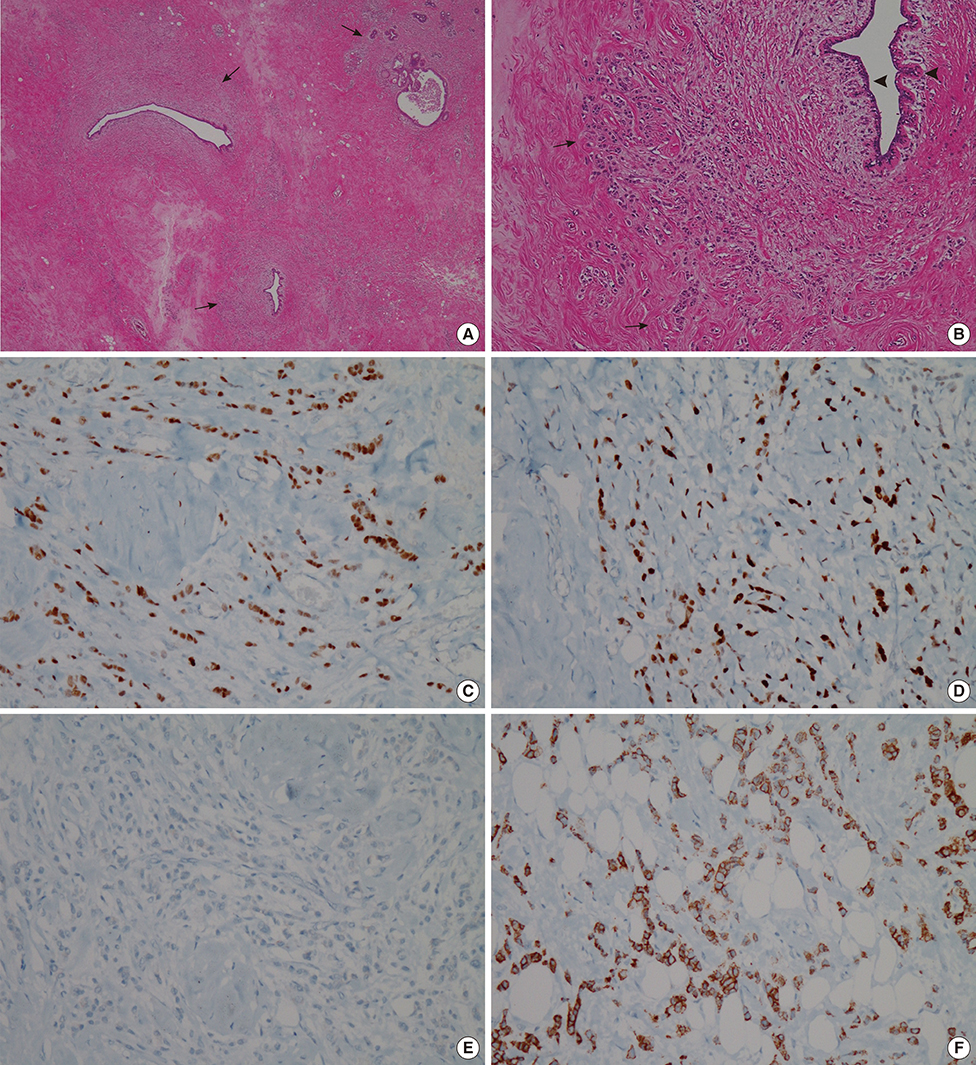
Figure
Reference
-
1. Wiernik PH, Hu X, Ratech H, Fineberg S, Marino P, Schleider MA, et al. Non-Hodgkin's lymphoma in women with breast cancer. Cancer J. 2000; 6:336–342.2. Dutta Roy S, Stafford JA, Scally J, Selvachandran SN. A rare case of breast carcinoma co-existing with axillary mantle cell lymphoma. World J Surg Oncol. 2003; 1:27.3. Suresh Attili VS, Dadhich HK, Rao CR, Bapsy PP, Batra U, Anupama G, et al. A case of breast cancer coexisting with B-cell follicular lymphoma. Austral-Asian J Cancer. 2007; 6:155–156.4. Benoit L, Arnould L, Collin F, Fraisse J, Cuisenier J, Chauffert B. Concurrent lymphoma and metastatic breast carcinoma in the axillary, confounding sentinel lymph-node biopsy. Eur J Surg Oncol. 2004; 30:462–463.
Article5. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168.6. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25:118–145.
Article7. Barranger E, Marpeau O, Uzan S, Antoine M. Axillary sentinel node involvement by breast cancer coexisting with B-cell follicular lymphoma in nonsentinel nodes. Breast J. 2005; 11:227–228.
Article8. Pandey U, Naraynan M, Karnik U, Sinha B. Carcinoma metastasis to unexpected synchronous lymphoproliferative disorder: report of three cases and review of literature. J Clin Pathol. 2003; 56:970–971.
Article9. Todd MT, Douglas Y. Bilateral breast cancer. In : Singletary SE, Robb GL, Hortobagyi GN, editors. Advanced Therapy of Breast Disease. 2nd ed. Hamilton: B.C. Decker;2004. p. 629–630.10. Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000; 19:257–262.
Article11. Gupta D, Merino MI, Farhood A, Middleton LP. Metastases to breast simulating ductal carcinoma in situ: report of two cases and review of the literature. Ann Diagn Pathol. 2001; 5:15–20.
Article12. MacNeill M, Arnott I, Thomas J. Fine needle aspiration cytology is a valuable adjunct to axillary ultrasound in the preoperative staging of breast cancer. J Clin Pathol. 2011; 64:42–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multifocal Bilateral Breast Cancer and Breast Follicular Lymphoma: A Simple Coincidence?
- Synchronous Presentation of Ductal Carcinoma In Situ of the Breast with Follicular Lymphoma
- Invasive Ductal Carcinoma of the Male Breast: A Case Report and Review of the Literature
- Nodular Metastatic Carcinoma from Invasive Lobular Breast Cancer
- Multiple Primary Malignant Neoplasms: A Case Report of Breast Mucinous Carcinoma and Extramammary Diffuse Large B-Cell Lymphoma in a Male Patient