Ann Dermatol.
2015 Jun;27(3):283-290. 10.5021/ad.2015.27.3.283.
Usefulness of Skin Explants for Histologic Analysis after Fractional Photothermolysis
- Affiliations
-
- 1Department of Dermatology, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea.
- 2Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csesnumd@gmail.com
- 3Asan Institute for Life Sciences, Seoul, Korea.
- KMID: 2352499
- DOI: http://doi.org/10.5021/ad.2015.27.3.283
Abstract
- BACKGROUND
Fractional laser resurfacing treatment has been extensively investigated and is widely used. However, the mechanism underlying its effects is poorly understood because of the ethical and cosmetic problems of obtaining skin biopsies required to study the changes after laser treatment.
OBJECTIVE
To evaluate the usefulness of human skin explants for the investigation of fractional photothermolysis.
METHODS
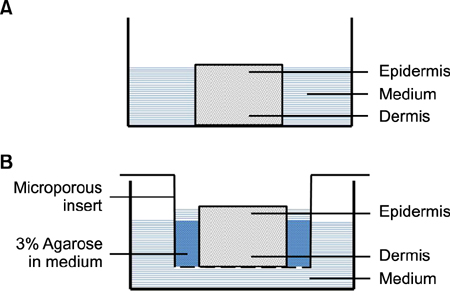
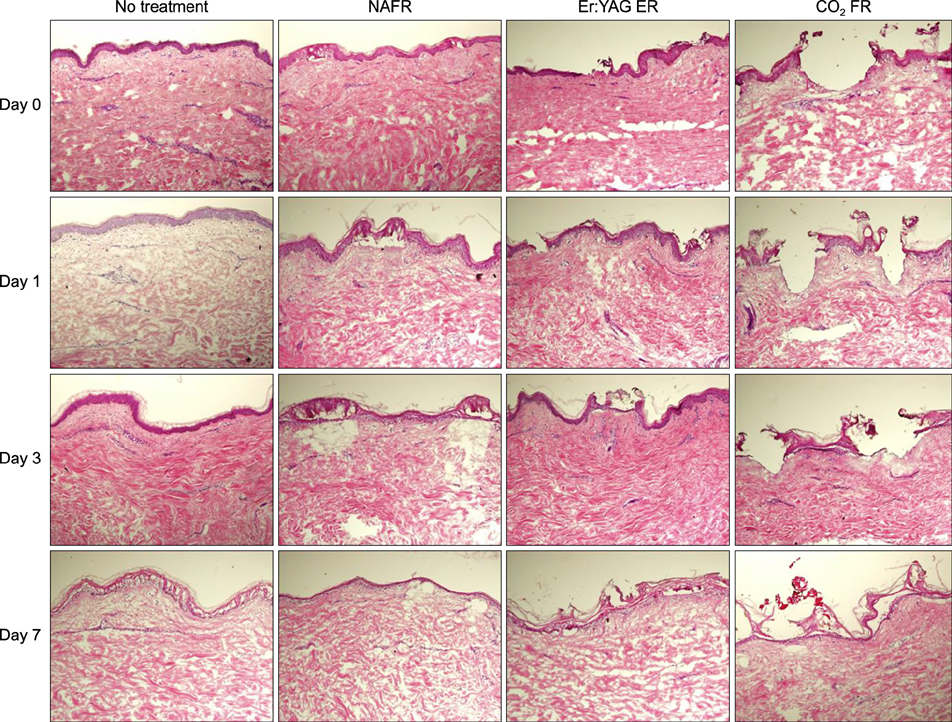
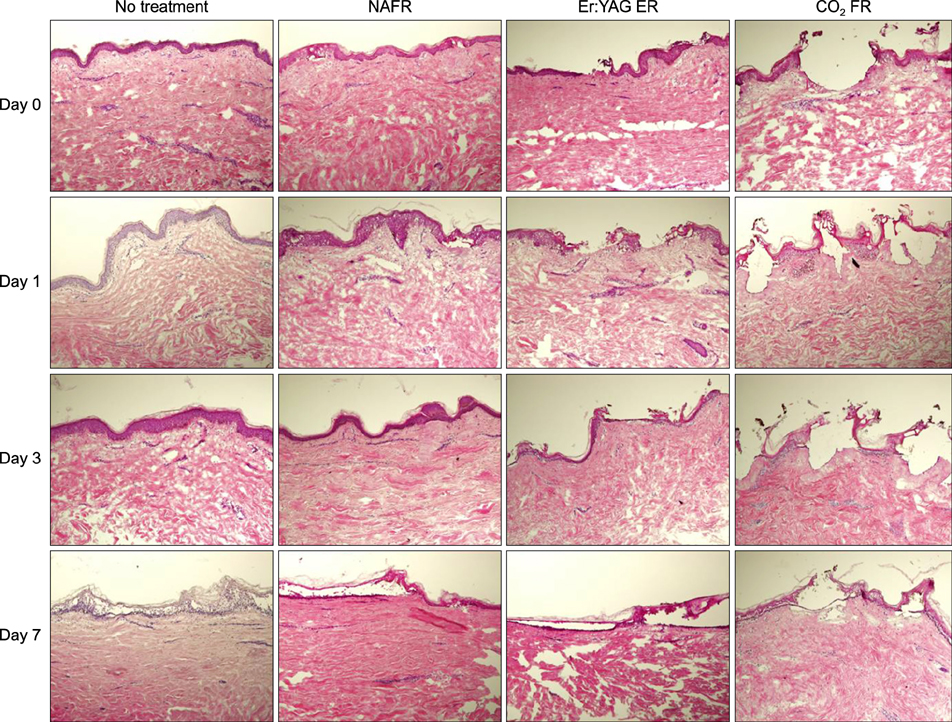
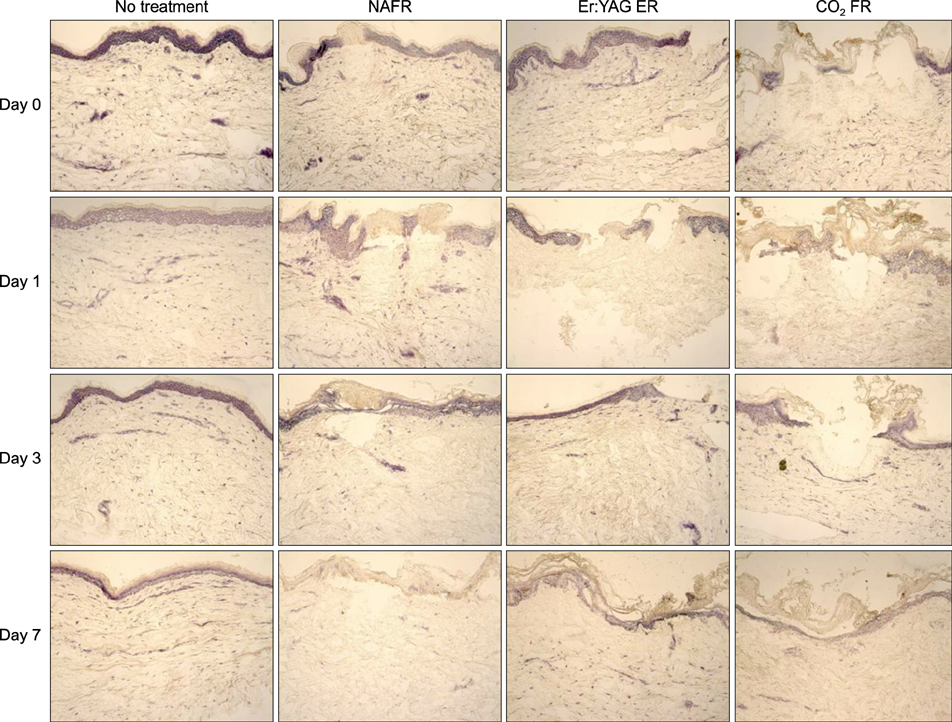
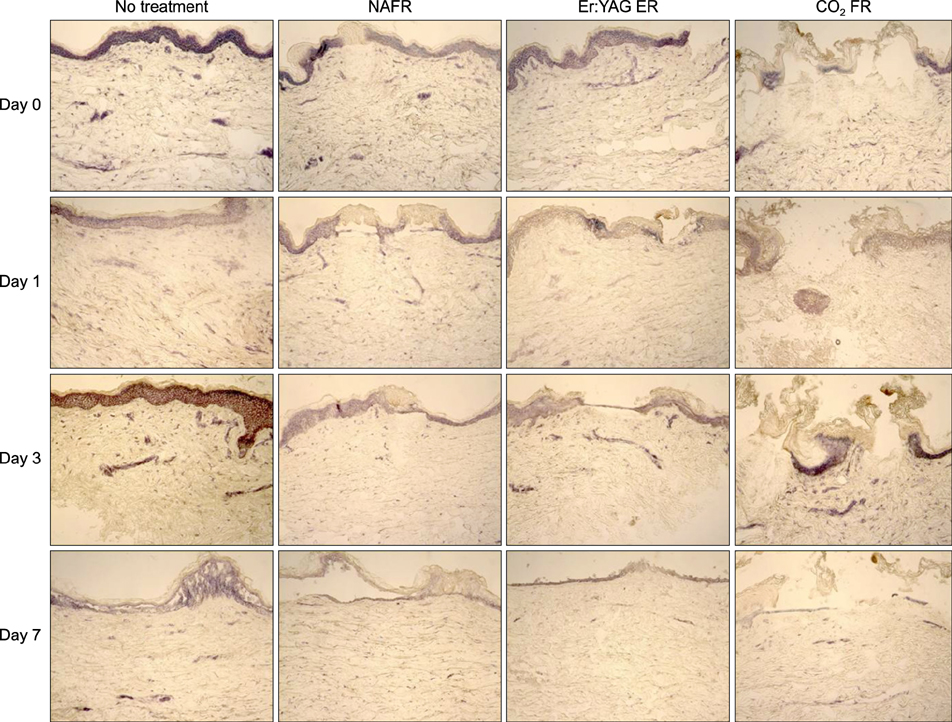
Full-thickness discarded skin was treated in 4 ways: no treatment (control), fractional carbon dioxide laser, fractional Er:YAG laser, and fractional 1,550-nm erbium-doped fiber laser. Both treated and non-treated skin samples were cultured ex vivo at the air-medium interface for 7 days. Frozen tissue was sectioned and stained with hematoxylin & eosin for histologic examination and nitro blue tetrazolium chloride for viability testing.
RESULTS
Skin explants cultured for up to 3 days exhibited histologic changes similar to those observed in in vivo studies, including microscopic treatment zones surrounded by a thermal coagulation zone, re-epithelialization, and formation of microscopic epidermal necrotic debris. However, the explant structure lost its original form within 7 days of culture. The viability of skin explants was maintained for 3 days of culture but was also lost within 7 days.
CONCLUSION
The skin explant model may be a useful tool for investigating the immediate or early changes following fractional photothermolysis, but further improvements are required to evaluate the long-term and dermal changes.
MeSH Terms
Figure
Reference
-
1. Skovbølling Haak C, Illes M, Paasch U, Hædersdal M. Histological evaluation of vertical laser channels from ablative fractional resurfacing: an ex vivo pig skin model. Lasers Med Sci. 2011; 26:465–471.
Article2. Walgrave S, Zelickson B, Childs J, Altshuler G, Erofeev A, Yaroslavsky I, et al. Pilot investigation of the correlation between histological and clinical effects of infrared fractional resurfacing lasers. Dermatol Surg. 2008; 34:1443–1453.
Article3. Swindle MM. Porcine integumentary system models: Part 1-Dermal toxicology [Internet]. Columbia: Sinclair Research Center Inc.;2008. updated 2008 Dec 16. cited 2013 Sep 25. Available at: http://www.sinclairbioresources.com/Downloads/TechnicalBulletins/Porcine%20Integumentary%20System%20Model-Part%201.pdf .4. Lee GH, Choe BI, Kim JS, Hart LA, Han JS. The current status of animal use and alternatives in Korean veterinary medical schools. Altern Lab Anim. 2010; 38:221–230.
Article5. Lebonvallet N, Jeanmaire C, Danoux L, Sibille P, Pauly G, Misery L. The evolution and use of skin explants: potential and limitations for dermatological research. Eur J Dermatol. 2010; 20:671–684.6. Companjen AR, van der Wel LI, Wei L, Laman JD, Prens EP. A modified ex vivo skin organ culture system for functional studies. Arch Dermatol Res. 2001; 293:184–190.
Article7. Joshi K, Demir H, Yamada R, Miyazaki T, Ray-Chaudhury A, Nakano I. Method for novel anti-cancer drug development using tumor explants of surgical specimens. J Vis Exp. 2011; (53):
Article8. Varani J, Fligiel SE, Schuger L, Perone P, Inman D, Griffiths CE, et al. Effects of all-trans retinoic acid and Ca++ on human skin in organ culture. Am J Pathol. 1993; 142:189–198.9. Eaton LH, Chularojanamontri L, Ali FR, Theodorakopoulou E, Dearman RJ, Kimber I, et al. Guttate psoriasis is associated with an intermediate phenotype of impaired Langerhans cell migration. Br J Dermatol. 2014; 171:409–411.
Article10. Lebonvallet N, Pennec JP, Le Gall-Ianotto C, Chéret J, Jeanmaire C, Carré JL, et al. Activation of primary sensory neurons by the topical application of capsaicin on the epidermis of a re-innervated organotypic human skin model. Exp Dermatol. 2014; 23:73–75.
Article11. Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J. 2013; 10:Suppl 1. 48–55.
Article12. Bedi VP, Chan KF, Sink RK, Hantash BM, Herron GS, Rahman Z, et al. The effects of pulse energy variations on the dimensions of microscopic thermal treatment zones in nonablative fractional resurfacing. Lasers Surg Med. 2007; 39:145–155.
Article13. Hantash BM, Bedi VP, Kapadia B, Rahman Z, Jiang K, Tanner H, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007; 39:96–107.
Article14. Jung KE, Jung KH, Park YM, Lee JY, Kim TY, Kim HO, et al. A split-face comparison of ablative fractional lasers (CO(2) and Er:YAG) in Asian patients; postprocedure erythema, pain and patient's satisfaction. J Cosmet Laser Ther. 2013; 15:70–73.
Article15. Lee HM, Haw S, Kim JE, Won CH, Lee MW, Choi JH, et al. A fractional 2940 nm short-pulsed, erbium-doped yttrium aluminium garnet laser is effective and minimally invasive for the treatment of photodamaged skin in Asians. J Cosmet Laser Ther. 2012; 14:253–259.
Article16. Foitzik K, Paus R, Doetschman T, Dotto GP. The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol. 1999; 212:278–289.
Article17. Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000; 6:475–479.
Article18. Neumann RA, Knobler RM, Pieczkowski F, Gebhart W. Enzyme histochemical analysis of cell viability after argon laser-induced coagulation necrosis of the skin. J Am Acad Dermatol. 1991; 25:991–998.
Article19. Steinstraesser L, Rittig A, Gevers K, Sorkin M, Hirsch T, Kesting M, et al. A human full-skin culture system for interventional studies. Eplasty. 2009; 9:e5.20. Xu W, Jong Hong S, Jia S, Zhao Y, Galiano RD, Mustoe TA. Application of a partial-thickness human ex vivo skin culture model in cutaneous wound healing study. Lab Invest. 2012; 92:584–599.
Article21. Helbig D, Moebius A, Simon JC, Paasch U. Nonablative skin rejuvenation devices and the role of heat shock protein 70: results of a human skin explant model. J Biomed Opt. 2010; 15:038002.
Article22. Alster TS, West TB. Resurfacing of atrophic facial acne scars with a high-energy, pulsed carbon dioxide laser. Dermatol Surg. 1996; 22:151–154. discussion 154-155.
Article23. Bernstein LJ, Kauvar AN, Grossman MC, Geronemus RG. Scar resurfacing with high-energy, short-pulsed and flashscanning carbon dioxide lasers. Dermatol Surg. 1998; 24:101–107.
Article24. Cotton J, Hood AF, Gonin R, Beesen WH, Hanke CW. Histologic evaluation of preauricular and postauricular human skin after high-energy, short-pulse carbon dioxide laser. Arch Dermatol. 1996; 132:425–428.
Article25. Kauvar AN, Waldorf HA, Geronemus RG. A histopathological comparison of "char-free" carbon dioxide lasers. Dermatol Surg. 1996; 22:343–348.
Article26. Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. An evaluation of 500 patients. Dermatol Surg. 1998; 24:315–320.
Article27. Ross EV, McKinlay JR, Anderson RR. Why does carbon dioxide resurfacing work? A review. Arch Dermatol. 1999; 135:444–454.28. Nagy IZ, Tóth VN, Verzár F. High-resolution electron microscopy of thermal collagen denaturation in tail tendons of young, adult and old rats. Connect Tissue Res. 1974; 2:265–272.
Article29. Ross EV, Naseef GS, McKinlay JR, Barnette DJ, Skrobal M, Grevelink J, et al. Comparison of carbon dioxide laser, erbium:YAG laser, dermabrasion, and dermatome: a study of thermal damage, wound contraction, and wound healing in a live pig model: implications for skin resurfacing. J Am Acad Dermatol. 2000; 42:92–105.
Article30. Verzár F, Nagy IZ. Electronmicroscopic analysis of thermal collagen denaturation in rat tail tendons. Gerontologia. 1970; 16:77–82.
Article31. Alster T, Zaulyanov L. Laser scar revision: a review. Dermatol Surg. 2007; 33:131–140.
Article32. Tanzi EL, Alster TS. Single-pass carbon dioxide versus multiple-pass Er:YAG laser skin resurfacing: a comparison of postoperative wound healing and side-effect rates. Dermatol Surg. 2003; 29:80–84.
Article33. Xu XG, Luo YJ, Wu Y, Chen JZ, Xu TH, Gao XH, et al. Immunohistological evaluation of skin responses after treatment using a fractional ultrapulse carbon dioxide laser on back skin. Dermatol Surg. 2011; 37:1141–1149.
Article34. Dierickx CC, Khatri KA, Tannous ZS, Childs JJ, Cohen RH, Erofeev A, et al. Micro-fractional ablative skin resurfacing with two novel erbium laser systems. Lasers Surg Med. 2008; 40:113–123.
Article35. Kist DA, Elm CM, Eleftheriou LI, Studer JA, Wallander ID, Walgrave SE, et al. Histologic analysis of a 2,940 nm fractional device. Lasers Surg Med. 2011; 43:79–91.36. Trelles MA, Vélez M, Mordon S. Correlation of histological findings of single session Er:YAG skin fractional resurfacing with various passes and energies and the possible clinical implications. Lasers Surg Med. 2008; 40:171–177.
Article37. Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004; 34:426–438.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Skin Characteristics after Fractional Photothermolysis
- Fractional Photothermolysis for Treatment of a Residual Discoid Lupus Erythematosus Lesion: A Case Report
- The Consideration of Dermoscopic Findings during Atrophic Acne Scar Treatment: a Pilot Study
- Treatment of Striae Distensae with Nonablative Fractional Laser versus Ablative CO2 Fractional Laser: A Randomized Controlled Trial
- Fractional Laser Photothermolysis for Treatment of Facial Wrinkles in Asians