J Korean Med Sci.
2015 Oct;30(Suppl 1):S59-S66. 10.3346/jkms.2015.30.S1.S59.
Current Status of Therapeutic Strategies for Patent Ductus Arteriosus in Very-Low-Birth-Weight Infants in Korea
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Boramae Hospital, Seoul, Korea.
- 2Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Dong-A University College of Medicine, Busan, Korea.
- 4Department of Biostatistics, Seoul National University Boramae Hospital, Seoul, Korea.
- 5Department of Pediatrics, Korea University College of Medicine, Seoul, Korea. cbmin@korea.ac.kr
- KMID: 2351137
- DOI: http://doi.org/10.3346/jkms.2015.30.S1.S59
Abstract
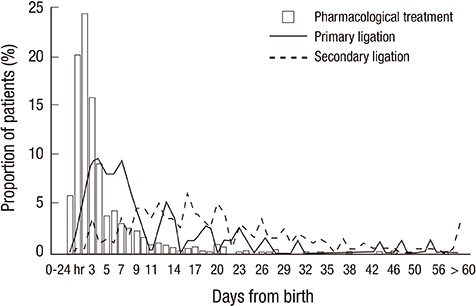
- This study aimed to investigate current therapeutic strategies for patent ductus arteriosus (PDA) in very-low-birth-weight (VLBW) infants in Korea. A total of 2,254 VLBW infants among 2,386 from Korean Neonatal Network cohort born from January 2013 to June 2014 were included. No PDA was seen for 1,206 infants (53.5%) and the infants diagnosed or treated for PDA were 1,048 infants (46.5%). The proportion of infants with PDA was decreased according to the increase in gestational age (GA) and birthweight. Infants with PDA were divided into groups according to the therapeutic strategies of PDA: prophylactic treatment (PT, n = 69, 3.1%), pre-symptomatic treatment (PST, n = 212, 9.4%), symptomatic treatment (ST, n = 596, 26.4%), and conservative treatment (CT, n = 171, 7.6%). ST was the most preferred treatment modality for preterm PDA and the proportion of the patients was decreased in the order of PST, CT, and PT. Although ST was still the most favored treatment in GA < 24 weeks group, CT was more preferred than PST or ST when compared with GA > or = 32 weeks group [CT vs. PST, OR 5.3, 95% CI 1.56-18.18; CT vs. ST, OR 2.9, 95% CI 1.03-8.13]. A total of 877 infants (38.9%) received pharmacological or surgical treatment about PDA, and 35.5% (801 infants) received pharmacological treatment, mostly with ibuprofen. Seventy-six infants (3.4%) received primary ligation and 8.9% (201 infants) received secondary ligation. Diverse treatment strategies are currently used for preterm PDA in Korea. Further analyses of neonatal outcomes according to the treatment strategies are necessary to obtain a standardized treatment guideline for preterm PDA.
MeSH Terms
Figure
Cited by 2 articles
-
Changes in neonatal outcomes in Korea
So Young Kim
J Korean Med Assoc. 2016;59(7):498-505. doi: 10.5124/jkma.2016.59.7.498.The Comparison of Efficiency of Oral Ibuprofen and Intravenous Indomethacin for Patent Ductus Arteriosus in Very Low Birth Weight Infants
Jong Woan Yun, Hyun A Park, Jong Hee Hwang
Perinatology. 2018;29(1):33-38. doi: 10.14734/PN.2018.29.01.33.
Reference
-
1. Madan JC, Kendrick D, Hagadorn JI, Frantz ID 3rd. National Institute of Child Health and Human Development Neonatal Research Network. Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics. 2009; 123:674–681.2. Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, Sekar K. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009; 123:e138–e144.3. Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O'Shea TM. Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics. 1999; 104:1345–1350.4. Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996; 75:F183–F186.5. Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005; 40:184–188.6. Heuchan AM, Clyman RI. Managing the patent ductus arteriosus: current treatment options. Arch Dis Child Fetal Neonatal Ed. 2014; 99:F431–F436.7. Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. 1983; 102:895–906.8. Benitz WE. Patent ductus arteriosus: to treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012; 97:F80–F82.9. Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. 2007; 27:164–170.10. Chorne N, Leonard C, Piecuch R, Clyman RI. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. 2007; 119:1165–1174.11. Alan S, Karadeniz C, Okulu E, Kilic A, Erdeve O, Ucar T, Atasay B, Atalay S, Arsan S. Management of patent ductus arteriosus in preterm infants: clinical judgment might be a fair option. J Matern Fetal Neonatal Med. 2013; 26:1850–1854.12. Rolland A, Shankar-Aguilera S, Diomande D, Zupan-Simunek V, Boileau P. Natural evolution of patent ductus arteriosus in the extremely preterm infant. Arch Dis Child Fetal Neonatal Ed. 2015; 100:F55–F58.13. Chang YS, Ahn SY, Park WS. The establishment of the Korean Neonatal Network (KNN). Neonatal Med. 2013; 20:169–178.14. Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010; 125:e214–e224.15. Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012; 36:123–129.16. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010; 126:443–456.17. Mirea L, Sankaran K, Seshia M, Ohlsson A, Allen AC, Aziz K, Lee SK, Shah PS. Canadian Neonatal Network. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. 2012; 161:689–694.e1.18. Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, Canpolat FE, Dilmen U. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014; 164:510–514.e1.19. Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010; CD000174.20. Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011; CD004213.21. Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003; CD003745.22. Hoellering AB, Cooke L. The management of patent ductus arteriosus in Australia and New Zealand. J Paediatr Child Health. 2009; 45:204–209.23. Cherif A, Jabnoun S, Khrouf N. Oral ibuprofen in early curative closure of patent ductus arteriosus in very premature infants. Am J Perinatol. 2007; 24:339–345.24. Lai LS, McCrindle BW. Variation in the diagnosis and management of patent ductus arteriosus in premature infants. Paediatr Child Health. 1998; 3:405–410.25. Gudmundsdottir A, Johansson S, Håkansson S, Norman M, Källen K, Bonamy AK. Timing of pharmacological treatment for patent ductus arteriosus and risk of secondary surgery, death or bronchopulmonary dysplasia: a population-based cohort study of extremely preterm infants. Neonatology. 2015; 107:87–92.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aspirin vs. Indomethacin in the Treatment of Patent Ductus Arteriosus in Very Low Birth Weight Infants
- Recent Advance in Management of Patent Ductus Arteriosus in Extremely Low Birth Weight Infants
- Therapeutic Strategies for PDA in Prematurity (How to treat PDA? When to treat PDA?)
- Oral Ibuprofen versus Intravenous Indomethacin for the Treatment of Patent Ductus Arteriosus in Very Low Birth Weight Infants
- Indomethacin therapy in premature infants with patent ductus arteriosus