Ann Dermatol.
2016 Aug;28(4):438-443. 10.5021/ad.2016.28.4.438.
Efficacy of a Complex of 5-Aminolevulinic Acid and Glycyl-Histidyl-Lysine Peptide on Hair Growth
- Affiliations
-
- 1Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea. weonju@knu.ac.kr
- 2Department of Pharmaceutical Engimeering, College of Public Health and Welfare, Dongshin University, Naju, Korea.
- KMID: 2344813
- DOI: http://doi.org/10.5021/ad.2016.28.4.438
Abstract
- BACKGROUND
Pattern hair loss is a very common problem. Although effective therapeutics for the treatment of pattern hair loss have been used, novel therapeutic modalities are still required to enhance hair growth.
OBJECTIVE
We investigated the efficacy and safety of a complex (ALAVAX) of 5-aminolevulinic acid (5-ALA) and glycyl-histidyl-lysine (GHK) peptide for the treatment of pattern hair loss.
METHODS
Forty-five patients with male pattern hair loss were treated with ALAVAX 100 mg/ml (group A), ALAVAX 50 mg/ml (group B) or placebo (group C) once a day for 6 months. Total hair count, hair length, hair thickness, patient's assessment and adverse events were evaluated at month 1, 3, and 6.
RESULTS
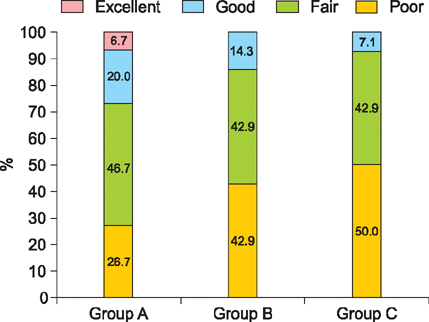
An increase in hair count for 6 months was 52.6 (p<0.05) in group A, 71.5 (p<0.05) in group B, and 9.6 in group C. The ratio of changes in hair count between group B (2.38) and group C (1.21) at 6 months showed a statistically significant difference (p<0.05). The proportion above good satisfaction was higher in group A (26.7%) than in the other groups (group B: 14.3%, group C: 7.1%). There was no statistically significant difference in hair length and hair thickness among 3 groups at 6 months. There was no adverse event in 3 groups.
CONCLUSION
Our study showed that a complex of 5-ALA and GHK peptide may be considered as one of the complementary agents for the treatment of male pattern hair loss.
Figure
Reference
-
1. Han SH, Byun JW, Lee WS, Kang H, Kye YC, Kim KH, et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann Dermatol. 2012; 24:311–318.
Article2. Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. J Drugs Dermatol. 2010; 9:1412–1419.3. Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, et al. Dutasteride Alopecia Research Team. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006; 55:1014–1023.
Article4. Munck A, Gavazzoni MF, Trüeb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014; 6:45–49.
Article5. Pyo HK, Yoo HG, Won CH, Lee SH, Kang YJ, Eun HC, et al. The effect of tripeptide-copper complex on human hair growth in vitro. Arch Pharm Res. 2007; 30:834–839.
Article6. Hostynek JJ, Dreher F, Maibach HI. Human skin penetration of a copper tripeptide in vitro as a function of skin layer. Inflamm Res. 2011; 60:79–86.
Article7. Pickart L. The human tri-peptide GHK and tissue remodeling. J Biomater Sci Polym Ed. 2008; 19:969–988.
Article8. Choi HR, Kang YA, Ryoo SJ, Shin JW, Na JI, Huh CH, et al. Stem cell recovering effect of copper-free GHK in skin. J Pept Sci. 2012; 18:685–690.
Article9. Wang H, Xu Y, Shi J, Gao X, Geng L. Photodynamic therapy in the treatment of basal cell carcinoma: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015; 31:44–53.
Article10. Asayama-Kosaka S, Akilov OE, Kawana S. Photodynamic therapy with 5% δ-aminolevulinic acid is safe and effective treatment of acne vulgaris in Japanese patients. Laser Ther. 2014; 23:115–120.
Article11. Jeong E, Hong JW, Min JA, Lee DW, Sohn MY, Lee WJ, et al. Topical ALA-photodynamic therapy for acne can induce apoptosis of sebocytes and down-regulate their TLR-2 and TLR-4 expression. Ann Dermatol. 2011; 23:23–32.
Article12. Morokuma Y, Yamazaki M, Maeda T, Yoshino I, Ishizuka M, Tanaka T, et al. Hair growth stimulatory effect by a combination of 5-aminolevulinic acid and iron ion. Int J Dermatol. 2008; 47:1298–1303.
Article13. Bissonnette R, Shapiro J, Zeng H, McLean DI, Lui H. Topical photodynamic therapy with 5-aminolaevulinic acid does not induce hair regrowth in patients with extensive alopecia areata. Br J Dermatol. 2000; 143:1032–1035.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A reliable method for the adjustment of urinary delta-aminolevulinic acid concentration
- The Effect of Calcitonin Gene-Related Peptide on Hair Growth in Vitro
- Hair follicle stem cells and mitochondria
- Expression of Protoporphyrin IX Induced by Liposome delta-5-aminolevulinic Acid in Cultured Sebocytes and Hair Organs
- Alopecia