J Korean Ophthalmol Soc.
2010 Dec;51(12):1549-1553.
Corneal Endothelial Change After Intravitreal Bevacizumab Injection
- Affiliations
-
- 1Department of Ophthalmology, Ewha Womans University School of Medicine, Seoul, Korea. jrmoph@ewha.ac.kr
Abstract
- PURPOSE
To evaluate the in vivo corneal endothelial changes after intravitreal bevacizumab (Avastin(R); Genentech Inc., San Francisco, California, USA) injection.
METHODS
A total of 30 eyes of 28 patients who received intravitreal bevacizumab injections were included in the present study. Before injection and one and three months after injection, specular microscopy was performed to analyze the corneal endothelial cell changes. In order to compare the differences in the changes of corneal endothelial cells, the eyes were divided into two groups, a single injection group and a multiple injection group.
RESULTS
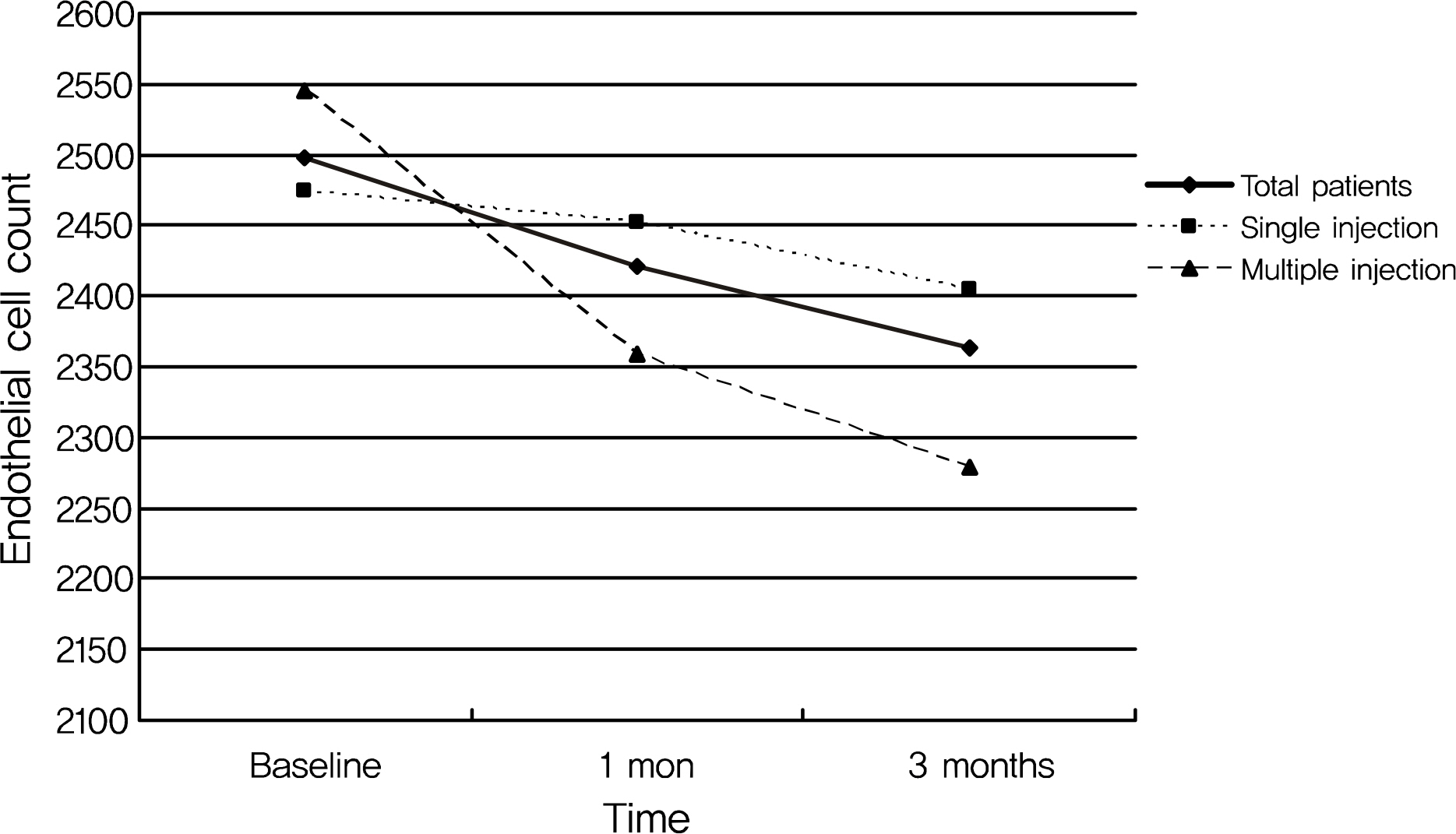
The mean endothelial cell count decreased from 2,497.4 +/- 427.8 at baseline to 2,421.2 +/- 430.5 at one month and to 2,362.7 +/- 366.2 at three months after the injection in all patients. However, the change in endothelial cell count was not statistically significant. In addition, the postoperative change in endothelial cell count was more prominent in the multiple injection group than in the single injection group, although the difference was again not significant. No significant changes in preoperative or postoperative coefficients of variation for cell area or hexagonalities were observed in either patient group or within each group.
CONCLUSIONS
There was no significant change in corneal endothelial cells after intravitreal bevacizumab injection. In addition, there was no significant difference in the changes in corneal endothelial cells according to the number of bevacizumab injections.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000; 41:2514–22.2. Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity due to soluble VEGF receptor-1. Nature. 2006; 443:993–7.3. Kvanta A, Algvere PV, Berglin L, et al. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996; 37:1929–34.
Article4. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for aberrations age-related macular degeneration. Ophthalmology. 2006; 113:363–72.5. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.
Article6. Wu L, Arevalo JF, Roca JA, et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative. Retina. 2008; 28:212–9.7. Destafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007; 125:834–6.
Article8. Grisanti S, Biester S, Peters S, et al. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006; 142:158–60.
Article9. Luthra S, Narayanan R, Marques LE, et al. Evaluation of in vitro effects of bevacizumab (avastin) on retinal pigment epithelial, neurosensory retinal, and microvascular endothelial cells. Retina. 2006; 26:512–8.
Article10. Yoeruek E, Spitzer MS, Aisenbey S, et al. Safety profile of bevacizumab on cultured human corneal cell. Cornea. 2007; 26:977–82.11. Spitzer MS, Wallenfels-Thilo B, Sierra A, et al. Antiproliferative and cytotoxic properties of bevacizumab in different ocular cells. Br J Ophthalmol. 2006; 90:1316–21.12. Chalam KV, Agarwal S, Brar VS, et al. Evaluation of cytotoxic effects of bevacizumab on human corneal cells. Cornea. 2009; 28:328–33.13. Park HY, Kim SJ, Lee HB, et al. Effect of intracameral bevacizumab injection on corneal endothelium in rabbits. Cornea. 2008; 27:1151–5.
Article14. Yoeruek E, Spitzer MS, Aisenbey S, et al. Safety profile of bevacizumab on cultured human corneal cell. Cornea. 2007; 26:977–82.15. Chiang CC, Chen WL, Lin JM, Tsai YY. Effect of bevacizumab on human corneal endothelial cells: a six-month follow up study. Am J Ophthalmol. 2008; 146:688–91.16. Hosny MH, Zayed MA, Shalaby AM, Eissa IM. Effect of intracameral bevacizumab injection on corneal endothelial cells: an in vivo evaluation. J Ocul Pharmacol Ther. 2009; 25:513–7.
Article17. Miyake T, Sawada O, Kakinoki M, et al. Pharmacokinetics of bevacizumab and its effect on vascular endothelial growth factor after intravitreal injection of bevacizumab in macaque eyes. Invest Ophthalmol Vis Sci. 2010; 51:1606–8.
Article18. Nomoto H, Shiraga F, Kuno N, et al. Pharmacokinetics of bevacizumab after topical, sub-conjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009; 50:4807–13.
Article19. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.
Article20. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single injection in humans. Am J Ophthalmol. 2008; 146:508–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Intravitreal Injection on the Corneal Endothelium
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- Corneal Endothelial Change After Intravitreal Triamcinolone Injection
- The Effect of Intravitreal Bevacizumab Injection in Wet Age-Related Macular Degeneration Patients with Cataract Surgery
- Effects of Intravitreal Injection of Bevacizumab or Ranibizumab on Systemic Circulation