J Korean Ophthalmol Soc.
2010 Aug;51(8):1039-1046.
Effects in Lumen Width of Nasolacrimal Drainage System After Adrenergic Drug Irrigation
- Affiliations
-
- 1Department of Ophthalmology, Seoul Veterans Hospital, Seoul, Korea. Ezer75@hanmail.net
Abstract
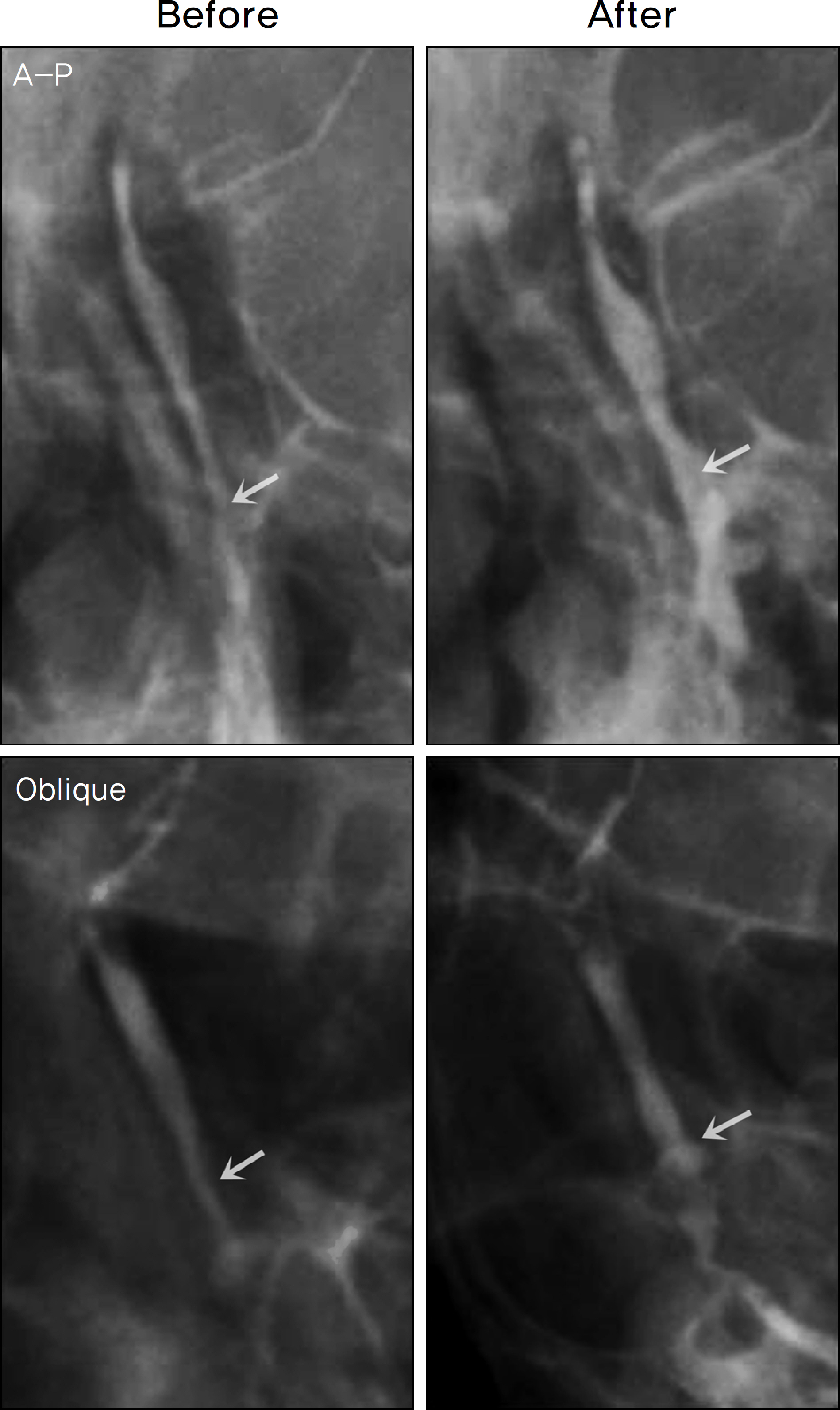
- PURPOSE
The lacrimal sac and nasolacrimal duct are surrounded by a wide cavernous system of veins and arteries, and the blood vessels of the cavernous body are innnervated by the autonomic nervous system. The purpose of this study was to determine the effect of an adrenergic agonist on the lumen width of the nasolacrimal drainage system.
METHODS
Dacryocystography was performed on 35 patients with only epiphora and not nasolacrimal duct obstruction. The anteroposterior (AP) diameters and the oblque diameters of the nasolacrimal ducts were measured. Next, 18 patients were infused with 0.5 ml Alphagan-P(R) (alpha-2 adrenergic receptor agonist), 17 patients were infused with 0.5 ml DL methylephedrine hydrochloride (alpha-1 and alpha-2 adrenergic receptor agonist), and dacryocystography was performed again to determine the change in the lumen width of the nasolacrimal drainage system.
RESULTS
The alpha-adrenergics caused a significant increase in the lumen width of the nasolacrimal drainage system, and the changes were more pronounced in the nasolacrimal duct than in the lacrimal sac. Although the nasolacrimal duct widening was more notable in the Alphagan-P(R) infusion group than the DL methylephedrine hydrochloride infusion group, there was no significant statistical difference. Patients' subjective symptoms improved in both groups.
CONCLUSIONS
The alpha-adrenergics constrict the blood vessels of the cavernous body, leading to the increase in the lumen width of the nasolacrimal drainage system. This effect was more significant in the Alphagan-P(R) infusion group. In conclusion, infusion of alpha-adrenergics in patients with functional nasolacrimal duct obstruction can be considered as an alternative to surgical management.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Thale A, Paulsen F, Rochels R, Tillmann B. Functional anatomy of the human efferent tear ducts: a new theory of tear outflow machanism. Graefes Arch Clin Exp Ophthalmol. 1998; 236:674–8.2. Paulsen F, Corfield A, Hinz M, et al. Characterization of mucins in human lacrimal sac and nasolacrimal duct. Invest Ophthalmol Vis Sci. 2003; 44:1807–13.
Article3. Paulsen F, Hinz M, Schaudig U, et al. TFF-peptides in the human efferent tear ducts. Invest Ophthalmol Vis Sci. 2002; 43:3359–64.4. Paulsen F. The human nasolacirmal ducts. Adv Anat Embryol Cell Biol. 2003; 170:1–106.5. Narioka J, Ohashi Y. Changes in lumen width of nasolacrimal drainage system after adrenergic and cholinergic stimulation. Am J Ophthalmol. 2006; 141:689–98.
Article6. Narioka J, Ohashi Y. Effects of adrenergic and cholinergic antago-nists on diameter of nasolacrimal drainage system. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1843–50.
Article7. Linberg JV, McCormick SA. Primary acquired nasolacrimal duct obstruction. A clinical pathologic report and biopsy technique. ophthalmology. 1986; 93:1055–63.8. Mauriello JA Jr, Palydowycz S, DeLuca J. Clinicopathologic study of lacrimal sac and nasal mucosa in 44 patients with complete acquired nasolacrimal duct obstruction. Ophthal Plast Reconstr Surg. 1992; 8:13–21.
Article9. Bartkey GB. Acquired lacrimal drainage obstruction: an eitologic classification system, case reports, and a review of the literature. Part 1. Ophthal Plast Reconstr Surg. 1992; 8:237–42.10. Summerskill WH. Problems of lacrimal obstruction; the rhinological approach. Trans Ophthalmol Soc UK. 1956; 76:385–7.11. Melanová J. Diverticulum of lacrimal sac [in Czech]. Ce Oftalmol. 1969; 25:47–8.12. Traquair HM. Chronic dacryocystitis; its causation and treatment. Arch Ophthalmol. 1941; 26:165–80.
Article13. Paulsen F, Hallmann U, Paulsen J, Thale A. Innervation of the cavernous body of the human efferent tear ducts and function in tear outflow mechanism. J Anat. 2000; 197:177–87.
Article14. Paulsen F, Thale A, Maune S, Tillmann B. New insight into the pathophysiology of Primary acquired dacryostenosis. Ophthalmology. 2001; 108:2329–36.15. Ayub M, Thale A, Heddeich J, et al. The cavernous body of the human efferent tear ducts contributes to regularion of tear outflow. Invest Ophthalmol Vis Sci. 2003; 44:4900–7.16. Henle J. Handbuch der Eingeweidelehre des Menschen. Braunschweig: Friedrich Vieweg;1866. p. 707–15.17. Dahl R, Mygind N. Anatomy, physiology and function of the nasal cavities in health and disease. Adv Drug Deliv Rev. 1998; 29:3–12.18. Thale A, Paulsen F, Rochles R, Tillmann B. Functional anatomy of human efferent tear ducts: a new theory of tear outflow mechanism. Graefes Arch Clin Exp Ophthalmol. 1998; 236:674–8.19. Joers K. Beiträge zur normalen und pathologischen Histologie des Tränenschlauches. Beiträge zur Augenheilk. 4:355–98.20. Paulsen F, Thale A, Kohla G, et al. Functioal anatomy of human lacrimal duct epithelium. Anat and Embryol. 1998; 198:1–12.21. Ishibe T, Yamashita T, Kumazawa T, Tanaka C. Adrenergic and cholinergic receptors in human nasal mucosa in cases of nasal allergy. Arch Otorhinolaryngol. 1983; 238:167–73.
Article22. Lindstrὃ m AE. Contribution to the knowledge of the incidence and treatment of the diseases of the lacrymal passages. Acta Ophthalmol. 1923; 1:131–46.23. Kawarai M, Koss MC. Sympathetic control of nasal blood flow in the rat mediated by alpha(1)-adrenoceptors. Eur J Pharmacol. 2001; 413:255–62.24. Corboz MR, Mutter JC, Rivelli MA, et al. Alpha2-adrenoceptor agonists as nasal decongestants. Pulm Pharmacol Ther. 2007; 20:149–56.25. Corboz MR, Rivelli MA, Mingo GG, et al. Mechanism of decon-gestant activity of alpha2-adrenoceptor agonists. Pulm Pharmacol Ther. 2008; 21:449–54.26. Brian B, Hoffman. Catecholamines, symphathomimetic drugs, and adrenergic receptor antagonist. Joel G. Hardman, Lee E. Limbird, Gilman Alfred Goodman, editors. The pharmacological basis of therapeutics. 10th ed.U.S.A.: McGraw-Hill;2001. v.1.chap. 10.27. Corboz MR, Rivelli MA, Varty LM, et al. Pharmacological characterization of postjunctional alpha-adrenoceptor in human nasal mucosa. Am J Rhinol. 2005; 19:495–502.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in the Lacrimal Excretory System Depending on the Administration Mode of Brimonidine Tartrate: Spray vs. Irrigation
- Results with Silicone Stent in Lacrimal Drainage System
- A Study on Incidence of Bacteriuria according to Bladder Irrigation in Patients with Indwelling Catheter
- Congenital nasolacrimal duct obstruction: irrigation or probing?
- Clinical Observation on Silicone Intubation in Obstruction of lacrimal Drainage System