J Korean Ophthalmol Soc.
2010 Jan;51(1):42-48.
Ultrastructure of the Internal Limiting Membrane Removed During Macular Hole and Diabetic Macular Edema Surgery
- Affiliations
-
- 1Department of Ophthalmology, School of Medicine, Pusan National University, Busan, Korea. bsoum@pusan.ac.kr
- 2Medical Research Institute, Pusan National University, Busan, Korea.
Abstract
- PURPOSE
To evaluate retinal damage following internal limiting membrane (ILM) peeling in macular hole and diabetic macular edema (DME) surgeries.
METHODS
Forty-five eyes with macular holes and thirty-five eyes with DME underwent pars plana vitrectomy with ILM peeling. The structures of the ILM were investigated using transmission electron microscopy, and the grades of retinal tissue damage were analyzed. We additionally observed the clinicopathologic association of retinal damage with the development of retinal hemorrhage during ILM peeling and that seen with indocyanine green (ICG) staining.
RESULTS
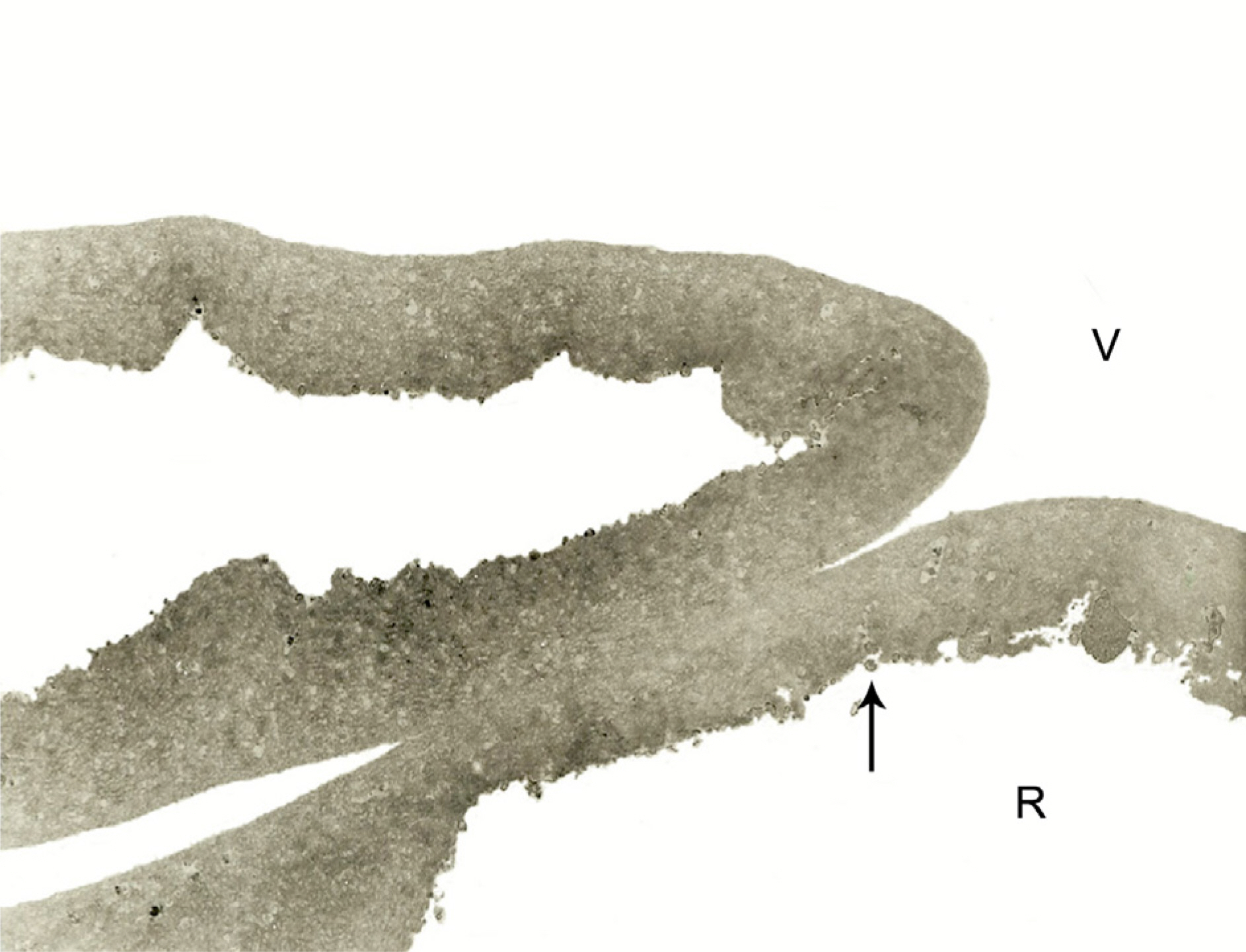
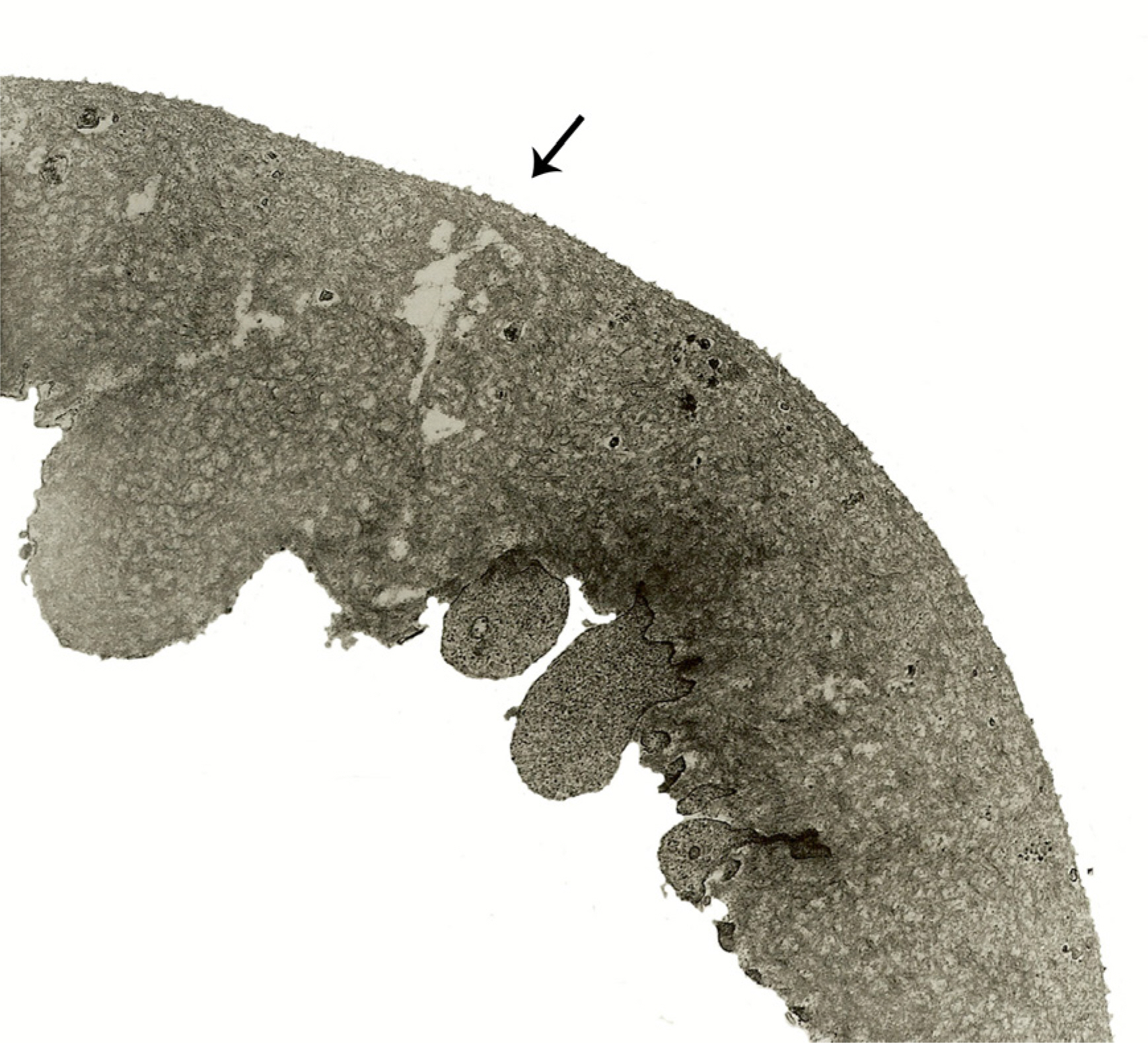
In all specimens, cellular fragments were observed on the retinal side of the ILM in both macular hole and DME patients. The thickness of the ILM in DME significantly increased (3.13+/-1.12 micrometer compared with that in patients with macular holes (2.41+/-0.77 micrometer, p=0.002). The frequency of minute retinal bleeding during ILM peeling was higher in macular hole patients (46.7%) than in those with diabetic macular edema (22.9%m p=0.028). Twenty-two eyes of 45 macular hole patients (48.9%) and 16 eyes of 35 DME patients (45.7%) had relative retinal damage. Overall, ILM performed in eyes which had minute bleeding during the peeling had more retinal damage (62.1%) than did those without hemorrhage (39.2%, p=0.049). ICG staining did not appear to influence retinal damage (p=0.81).
CONCLUSIONS
ILM peeling can cause minor, but demonstrable, damage of the adjacent retina.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Tognetto D, Grandin R, Sanguinetti G, et al. Internal limiting aberrations removal during macular hole surgery: results of a aberrations retrospective study. Ophthalmology. 2006; 113:1401–10.2. Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000; 20:126–33.
Article3. Matsunaga N, Ozeki H, Hirabayashi Y, et al. Histopathologic evaluation of the internal limiting membrane surgically excised from eyes with diabetic maculopathy. Retina. 2005; 25:311–6.
Article4. Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998; 82:561–8.
Article5. Barile GR, Pachydaki SI, Tari SR, et al. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005; 46:2916–24.
Article6. Uemoto R, Yamamoto S, Aoki T, et al. Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. Br J Ophthalmol. 2002; 86:1240–2.
Article7. Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006; 51:364–80.
Article8. Margherio RR, Margherio AR, Williams GA, et al. Effect of perifoveal tissue dissection in the management of acute idiopathic full-thickness macular holes. Arch Ophthalmol. 2000; 118:495–8.
Article9. Aboutable T. Is removal of internal limiting membrane always necessary during surgery for refractory diffuse diabetic macular edema without evident epimacular proliferation? Klin Monatsbl Augenheilkd. 2006; 223:681–6.10. Uemoto R, Yamamoto S, Takeuchi S. Changes in retinal pigment epithelium after indocyanine green-assisted internal limiting lamina peeling during macular hole surgery. Am J Ophthalmol. 2005; 140:752–5.
Article11. Ando F, Yasui O, Hirose H, Ohba N. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Adverse effect of ICG-assisted ILM peeling. Graefes Arch Clin Exp Ophthalmol. 2004; 242:995–9.12. Horio N, Horiguchi M. Effect on visual outcome after macular hole surgery when staining the internal limiting membrane with indocyanine green dye. Arch Ophthalmol. 2004; 122:992–6.13. Yamashita T, Uemura A, Kita H, Sakamoto T. Analysis of the retinal nerve fiber layer after indocyanine green assisted aberrations for idiopathic macular holes. Ophthalmology. 2006; 113:280–4.14. Gandorfer A, Haritoglou C, Gass CA, et al. Indocyanine aberrations peeling of the internal limiting membrane may cause aberrations damage. Am J Ophthalmol. 2001; 132:431–3.15. Schumann RG, Schaumberger MM, Rohleder M, et al. aberrations of the Vitreomacular Interface in Full-Thickness Idiopathic Macular Holes: A Consecutive Analysis of 100 Cases. Am J aberrations. 2006; 141:1112–9.16. Radetzky S, Walter P, Fauser S, et al. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004; 242:273–8.
Article17. Halfter W, Reckhaus W, Kröger S. Nondirected axonal growth on basal lamina from avian embryonic neural retina. J Neurosci. 1987; 7:3712–22.
Article18. Newman EA. Regional specialization of retinal glial cell aberrations. Nature. 1984; 309:155–7.19. Winter M, Eberhardt W, Scholz C, Reichenbach A. Failure of potassium siphoning by Müller cells: a new hypothesis of perfluorocarbon liquid-induced retinopathy. Invest Ophthalmol Vis Sci. 2000; 41:256–61.20. Wolf S, Schnurbusch U, Wiedemann P, et al. Peeling of the basal membrane in the human retina: ultrastructural effects. aberrationsogy. 2004; 111:238–43.21. Mitamura Y, Ohtsuka K. Relationship of dissociated optic nerve fiber layer appearance to internal limiting membrane peeling. Ophthalmology. 2005; 112:1766–70.
Article22. Kuhn F, Morris R, Mester V, Witherspoon CD. Internal limiting membrane removal for traumatic macular holes. Ophthalmic Surg Lasers. 2001; 32:308–15.
Article23. Nakamura T, Murata T, Hisatomi T, et al. Ultrastructure of the vitreoretinal interface following the removal of the internal limiting membrane using indocyanine green. Curr Eye Res. 2003; 27:395–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eccentric Macular Hole Formation After Macular Hole Surgery
- Effect of Removal of Internal Limiting Membrane in Macular Hole Surgery
- Influence of the Macular Curvature on Foveal Migration after Macular Hole Surgery
- Effect of Internal Limiting Membrane Peeling in Idiopathic Macular Holes Stage 3, 4
- Effect of Internal Limiting Membrane Removal in Treatment of Retinal Detachment Caused by Myopic Macular Hole