J Korean Orthop Assoc.
2009 Apr;44(2):151-158.
Glucocorticoid-induced Osteoporosis; Update
- Affiliations
-
- 1Skeletal Diseases Genome Research Cener, School of Medicine, Kyungpook National University Hospital, Daegu, Korea. biohjk@knu.ac.kr
Abstract
- No abstract available.
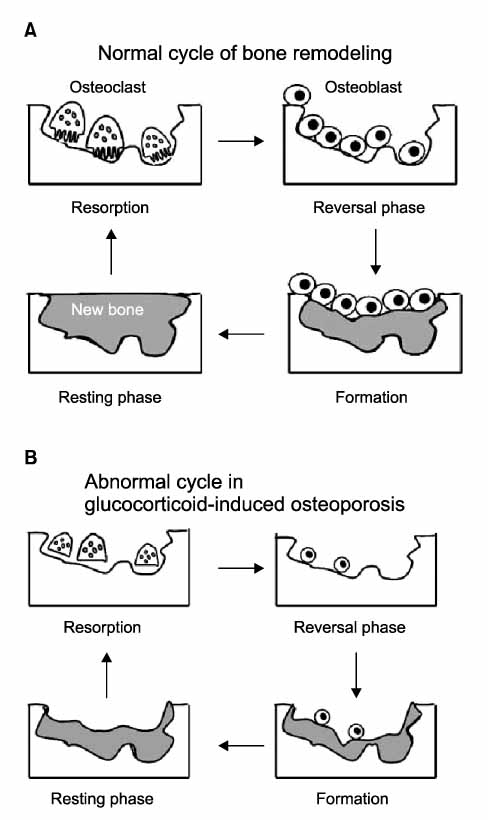
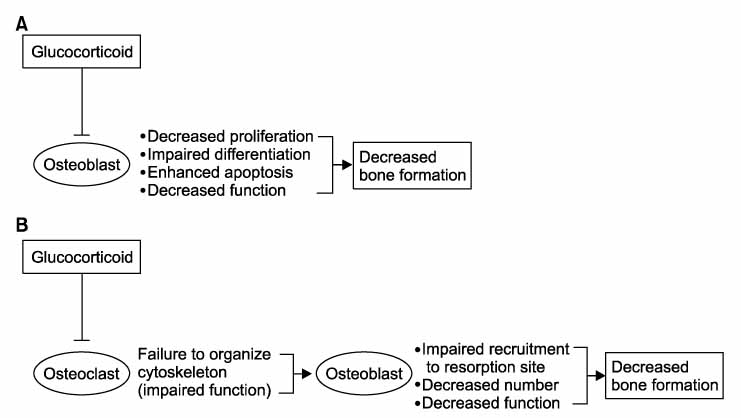
Figure
Reference
-
1. Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 1999. 72:396–410.
Article2. Bikle DD, Halloran B, Fong L, Steinbach L, Shellito J. Elevated 1,25-dihydroxyvitamin D levels in patients with chronic obstructive pulmonary disease treated with prednisone. J Clin Endocrinol Metab. 1993. 76:456–461.
Article3. Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003. 349:1207–1215.
Article4. Canalis E. Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996. 81:3441–3447.5. Canalis E, Bilezikian JP, Angeli A, Giustina A. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004. 34:593–598.
Article6. Chellaiah MA, Soga N, Swanson S, et al. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 2000. 275:11993–12002.
Article7. Dalle Carbonare L, Arlot ME, Chavassieux PM, Roux JP, Portero NR, Meunier PJ. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J Bone Miner Res. 2001. 16:97–103.
Article8. D'Amelio P, Grimaldi A, Di Bella S, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008. 43:92–100.9. Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J Cell Biol. 2003. 162:499–509.10. Faccio R, Teitelbaum SL, Fujikawa K, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005. 11:284–290.
Article11. Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003. 349:1216–1226.
Article12. Hattersley AT, Meeran K, Burrin J, Hill P, Shiner R, Ibbertson HK. The effect of long- and short-term corticosteroids on plasma calcitonin and parathyroid hormone levels. Calcif Tissue Int. 1994. 54:198–202.13. Kim HJ, Zhao H, Kitaura H, et al. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006. 116:2152–2160.
Article14. Klein RG, Arnud SB, Gallagher JC, Deluca HF, Riggs BL. Intestinal calcium absorption in exogenous hypercortisonism. Role of 25-hydroxyvitamin D and corticosteroid dose. J Clin Invest. 1997. 60:253–259.15. Krum SA, Miranda-Carboni GA, Hauschka PV, et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008. 27:535–545.
Article16. Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996. 55:288–293.
Article17. Luengo M, Picado C, Piera C, et al. Intestinal calcium absorption and parathyroid hormone secretion in asthmatic patients on prolonged oral or inhaled steroid treatment. Eur Respir J. 1991. 4:441–444.18. Lukert B, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and managemnt. Ann Intern Med. 1990. 112:352–364.19. Monder C, Miroff Y, Marandici A, Hardy MP. 11 beta-hydroxysteroid dehydrogenase allevates glucocorticoid-mediated inhibition of steroidogenesis in rat Leydig cells. Endocrinology. 1994. 134:1199–1204.20. Nakamura I, Kadono Y, Takayanagi H. IL-1 regulates cytoskeletal organization in osteoclasts via TNF receptor-associated factor 6/c-Src complex. J Immunol. 2002. 168:5103–5109.
Article21. Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun. 2005. 329:177–181.
Article22. Paz-pacheco E, Fuleihan GE, LeBoff MS. Intact parathyroid hormone levels are not eevated in glucocorticoid-treated subjects. J Bone Miner Res. 1995. 10:1713–1718.23. Pereira RC, Delany AM, Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone. 2002. 30:685–691.
Article24. Pereira RM, Delany AM, Canalis E. Cortisol inhibits the differentiation and apoptosis of osteoblasts in culture. Bone. 2001. 28:484–490.
Article25. Purpura KA, Aubin JE, Zandstra PW. Sustained in vitro expansion of bone progenitors is cell density dependent. Stem Cells. 2004. 22:39–50.
Article26. Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002. 365:561–575.
Article27. Reid IR. Glucocorticoid osteoporosis-mechanisms and management. Eur J Endocrinol. 1997. 137:209–217.
Article28. Smith E, Coetzee GA, Frenkel B. Glucocorticoids inhibit cell cycle progression in differentiating osteoblasts via glycogen synthase kinase-3beta. J Biol Chem. 2002. 277:18191–18197.29. Smith E, Frenkel B. Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3-beta-dependent and -independent manner. J Biol Chem. 2005. 280:2388–2394.30. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000. 289:1504–1508.
Article31. Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Path. 2007. 170:427–435.
Article32. Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord. 2001. 2:65–73.33. Weinstein RS, Chen JR, Powers CC, et al. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002. 109:1041–1048.
Article34. Weistein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechnisms of their deleterious effects on bone. J Clin Invest. 1998. 102:274–282.35. Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996. 16:4128–4136.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update on Glucocorticoid Induced Osteoporosis
- Glucocorticoid-induced Osteoporosis
- Understanding of Glucocorticoid Induced Osteoporosis
- Analysis on Management Status of Glucocorticoid Induced Osteoporosis in Patient with Rheumatoid Arthritis
- Management of glucocorticoid-related osteoporotic vertebral fracture