World J Mens Health.
2012 Dec;30(3):177-182.
Preoperative Factors Influencing Postoperative Results after Vasovasostomy
- Affiliations
-
- 1Department of Urology, Chonbuk National University Medical School, Jeonju, Korea. rain@chonbuk.ac.kr
- 2Institute for Medical Sciences, Chonbuk National University, Jeonju, Korea.
- 3Biomedical Research Institute and Clinical Trial Center of Medical Device, Chonbuk National University Hospital, Jeonju, Korea.
Abstract
- PURPOSE
The purpose of this study is to evaluate the preoperative factors that influenced postoperative sperm concentration after vasovasostomy.
MATERIALS AND METHODS
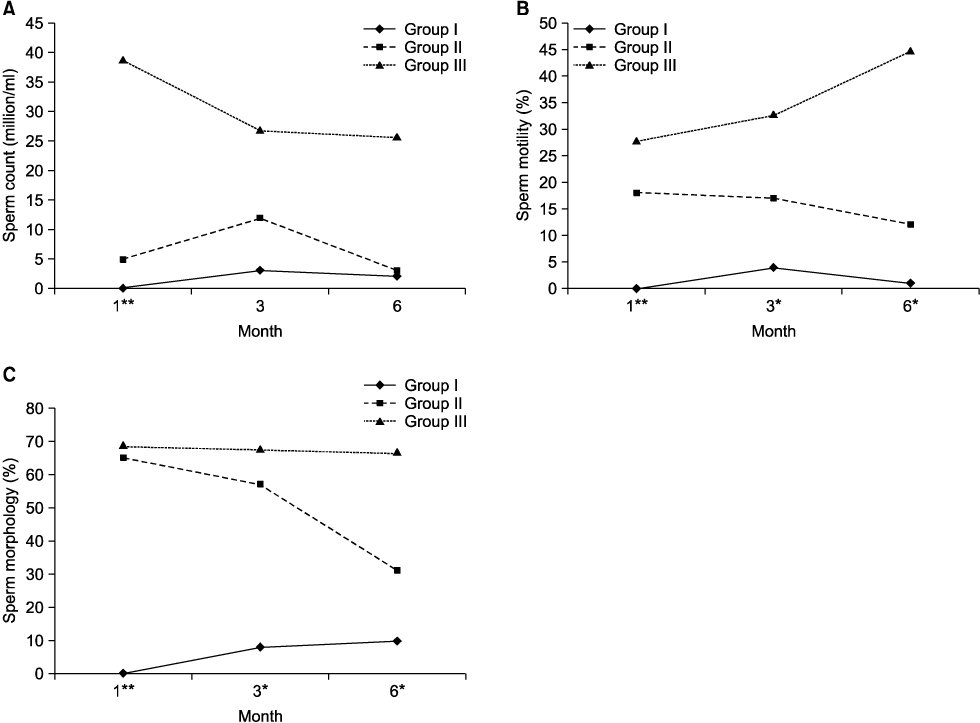
We retrospectively reviewed 97 consecutive single-layer vasovasostomy procedures performed by a single surgeon between March 2003 and September 2010. The patients were stratified into three groups based on sperm concentration at 1 month follow-up: group I-azoospermia, group II-oligospermia, and group III-normal. We evaluated the preoperative factors that may have influenced sperm concentration at postoperative 1 month. Patients with serial semen analysis were divided into four groups according to the change in postoperative sperm concentration at the 6-month visit: group II-N-from oligospermia to normal, group II-O-from oligospermia to oligospermia, group III-O-from normal to oligospermia, group III-N-from normal to normal. We compared the pregnancy rate among the four groups.
RESULTS
The mean obstructive interval was 9.69 years in group I, 6.02 years in group II, and 7.82 years in group III. There were significant differences found among the groups (p=0.035). There was significantly different change in sperm concentration, sperm motility, and sperm morphology between each of the groups. A total of 32 patients underwent serial semen analyses at 1 month, 3 months, and 6 months after vasovasostomy. There was no significant difference in patient age, obstructive interval, or follicle-stimulating hormone among the groups. The natural pregnancy rate in group II-O was lower than that in group II-N, and in group III-O was lower than that in group III-N. However, there was no significant difference among each of the groups.
CONCLUSIONS
The sperm concentration after vasovasostomy was significantly related to the obstructive interval between vasectomy and reversal.
Keyword
MeSH Terms
Figure
Reference
-
1. Weiske WH. Vasectomy. Andrologia. 2001. 33:125–134.
Article2. Boorjian S, Lipkin M, Goldstein M. The impact of obstructive interval and sperm granuloma on outcome of vasectomy reversal. J Urol. 2004. 171:304–306.
Article3. Lipshultz LI, Rumohr JA, Bennett RC. Techniques for vasectomy reversal. Urol Clin North Am. 2009. 36:375–382.
Article4. Silber SJ. Microscopic vasectomy reversal. Fertil Steril. 1977. 28:1191–1202.5. Cos LR, Valvo JR, Davis RS, Cockett AT. Vasovasostomy: current state of the art. Urology. 1983. 22:567–575.
Article6. Belker AM, Thomas AJ Jr, Fuchs EF, Konnak JW, Sharlip ID. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991. 145:505–511.
Article7. Matthews GJ, McGee KE, Goldstein M. Microsurgical reconstruction following failed vasectomy reversal. J Urol. 1997. 157:844–846.
Article8. Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol. 1998. 159:188–190.
Article9. Jokelainen OS, Rintala E, Koskimies AI, Rannikko S. Vasovasostomy-a 15-year experience. Scand J Urol Nephrol. 2001. 35:132–135.10. Holman CD, Wisniewski ZS, Semmens JB, Rouse IL, Bass AJ. Population-based outcomes after 28,246 in-hospital vasectomies and 1,902 vasovasostomies in Western Australia. BJU Int. 2000. 86:1043–1049.
Article11. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 2010. 5th ed. Geneva: WHO Press;15–44.12. Belker AM. Vasectomy reversal. Urol Clin North Am. 1987. 14:155–166.
Article13. Raleigh D, O'Donnell L, Southwick GJ, de Kretser DM, McLachlan RI. Stereological analysis of the human testis after vasectomy indicates impairment of spermatogenic efficiency with increasing obstructive interval. Fertil Steril. 2004. 81:1595–1603.
Article14. Fuchs EF, Burt RA. Vasectomy reversal performed 15 years or more after vasectomy: correlation of pregnancy outcome with partner age and with pregnancy results of in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2002. 77:516–519.
Article15. Kolettis PN, Woo L, Sandlow JI. Outcomes of vasectomy reversal performed for men with the same female partners. Urology. 2003. 61:1221–1223.
Article16. Chan PT, Goldstein M. Superior outcomes of microsurgical vasectomy reversal in men with the same female partners. Fertil Steril. 2004. 81:1371–1374.
Article17. Belker AM, Fuchs EF, Konnak JW, Sharlip ID, Thomas AJ Jr. Transient fertility after vasovasostomy in 892 patients. J Urol. 1985. 134:75–76.
Article18. Matthews GJ, Schlegel PN, Goldstein M. Patency following microsurgical vasoepididymostomy and vasovasostomy: temporal considerations. J Urol. 1995. 154:2070–2073.
Article19. Jarow JP, Sigman M, Buch JP, Oates RD. Delayed appearance of sperm after end-to-side vasoepididymostomy. J Urol. 1995. 153:1156–1158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors influencing the success rate of pregnancy following microscopic vasovasostomy for postvasectomy sterility
- Comparative evaluation about factors influencing the success rate of microscopic vasovasostomy
- The Incidence of Fever after Subinguinal Microsurgical Varicocelectomy
- Vasovasostomy: With Fine Suture Material Without Internal Stent
- The Results of Microscopic Vasovasostomies with Different Methods in the Vasectomized Patients