Lab Med Online.
2016 Jul;6(3):152-158. 10.3343/lmo.2016.6.3.152.
Comparing the Efficacy of Samsung LABGEO PT10 and Bio-Rad Variant II Turbo for HbA1c Measurement in Three Types of Blood Samples
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. nayadoo@hanmail.net
- KMID: 2308730
- DOI: http://doi.org/10.3343/lmo.2016.6.3.152
Abstract
- BACKGROUND
Hemoglobin A1c (HbA1c) is a good marker for monitoring glycemic control. The Samsung LABGEO PT10 HbA1c test (Samsung Electronics, Korea) was developed as a point-of-care testing approach. This study evaluated the levels of HbA1c in three different types of blood specimens using two different methods.
METHODS
We used correlation analyses to compare the results obtained using Samsung LABGEO PT10 and Bio-Rad Variant II Turbo (Bio-Rad Laboratories, USA) to determine the levels of HbA1c in three different types of blood samples: capillary blood, EDTA whole blood, and lithium (Li)-heparin whole blood.
RESULTS
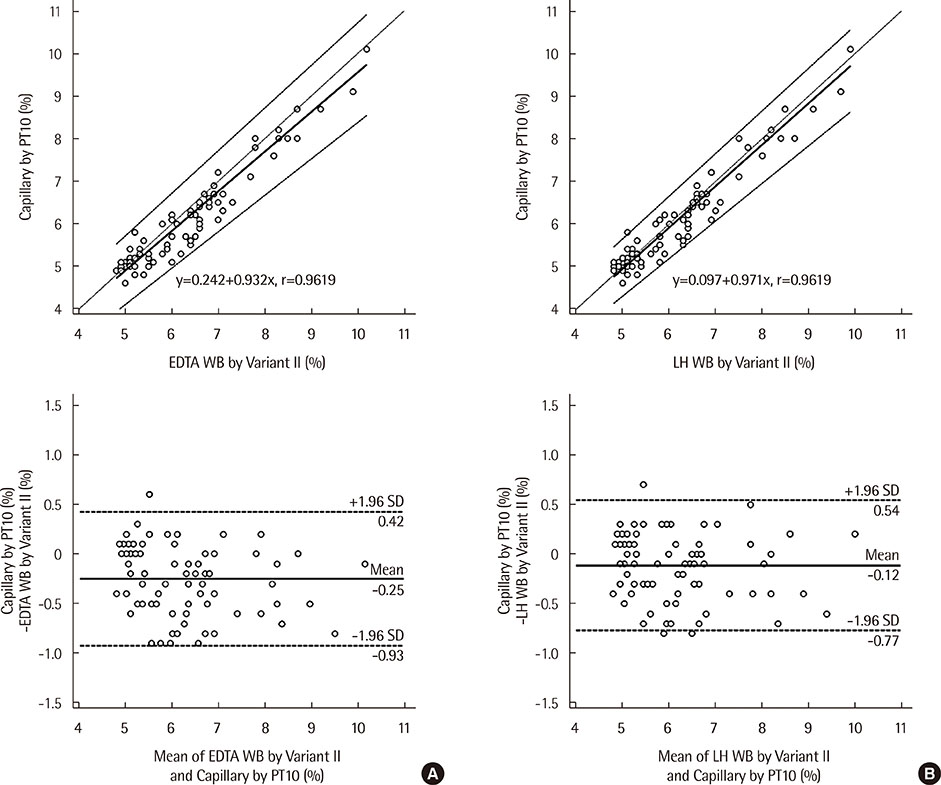
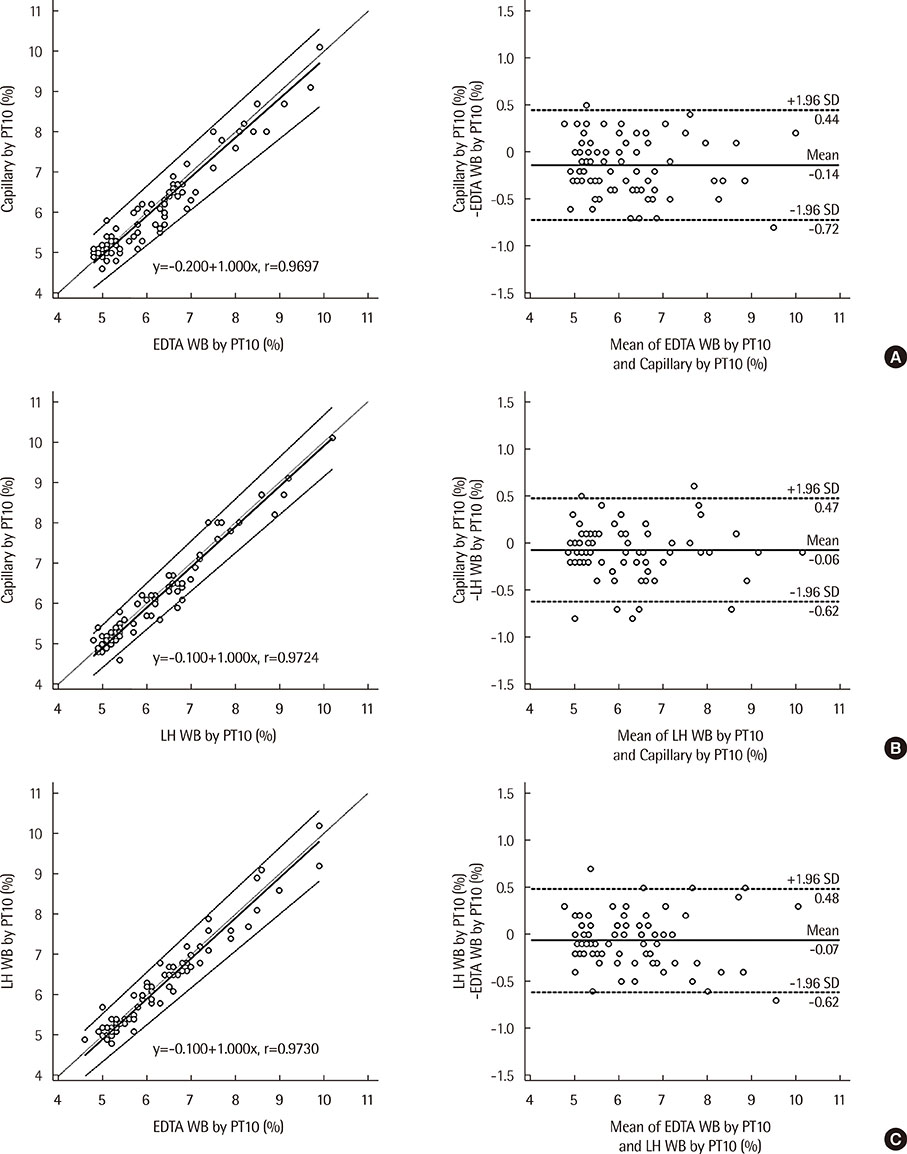
The correlation coefficient for the level of HbA1c in capillary blood based on LABGEO PT10 vs. that in EDTA whole blood based on the Variant II Turbo was r=0.9619; that in capillary blood based on LABGEO PT10 vs. that in Li-heparin whole blood based on the Variant II Turbo was r=0.9619; that in capillary blood vs. that in EDTA whole blood based on the LABGEO PT10 was r=0.9697; that in capillary blood vs. that in Li-heparin whole blood based on the LABGEO PT10 was r=0.9724; and that in EDTA whole blood vs. that in Li-heparin whole blood based on the LABGEO PT10 was r=0.9730.
CONCLUSIONS
The LABGEO PT10 was suitable for analyzing HbA1c. The results for the measurement of HbA1c levels in capillary blood were comparable to that in the whole blood samples. Additionally, LABGEO PT10 can be used for patients who are unable to take venipuncture.
Keyword
MeSH Terms
Figure
Reference
-
1. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329:977–986.2. World Health Organization. WHO diabetes media centre. Updated on Jan 2015. http://www.who.int/mediacentre/factsheets/fs312/en/index.html.3. Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer HM, Rohlfing C, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005; 51:753–758.
Article4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37:Suppl 1. S81–S90.5. St John A, Davis TM, Goodall I, Townsend MA, Price CP. Nurse-based evaluation of point-of-care assays for glycated haemoglobin. Clin Chim Acta. 2006; 365:257–263.
Article6. Miller CD, Barnes CS, Phillips LS, Ziemer DC, Gallina DL, Cook CB, et al. Rapid A1c availability improves clinical decision-making in an urban primary care clinic. Diabetes Care. 2003; 26:1158–1163.
Article7. Jeong TD, Lee W, Chun S, Min WK. Performance evaluation of the LABGEO PT10 point-of-care chemistry analyzer. J Lab Med Qual Assur. 2013; 35:70–80.8. Clinical and Laboratory Standards Institute. Measurement procedure comparison and bias estimation using patient samples: approved guideline. Wayne, PA: CLSI;2013.9. Clinical and Laboratory Standards Institute. Evaluation of matrix effects: approved guideline. Wayne, PA: Clinical and Laboratory Standards Institute;2005.10. Altman DG, editor. Practical statistics for medical research. London: New York: Chapman and Hall;1991.11. Solvik UO, Roraas T, Christensen NG, Sandberg S. Diagnosing diabetes mellitus: performance of hemoglobin A1c point-of-care instruments in general practice offices. Clin Chem. 2013; 59:1790–1801.
Article12. Weykamp C, John WG, Mosca A, Hoshino T, Little R, Jeppsson JO, et al. The IFCC Reference Measurement System for HbA1c: a 6-year progress report. Clin Chem. 2008; 54:240–248.
Article13. Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009; 3:446–451.
Article14. Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001; 47:153–163.
Article15. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011; 57:e1–e47.
Article16. Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010; 56:44–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the LABGEO PT10 Point-of-care Chemistry Analyzer
- Evaluation of HbA1c on COBAS INTEGRA 800 Closed Tube System Compared with Variant II Turbo
- Evaluation of the Performance of ARKRAY ADAMS HA-8180 HbA1c Analyzer
- Evaluation of the AutoLab HbA1c Reagent by Using Hitachi Clinical Analyzer 7180
- Evaluation of Cobas b 101 HbA1c Analyzer Performance for Point-of-Care Testing