Korean J Urol.
2006 Jun;47(6):607-613. 10.4111/kju.2006.47.6.607.
Correlation of Caveolin-1 Expression on Tissue Microarray with Prognosis in Renal Cell Carcinoma
- Affiliations
-
- 1Department of Urology, Ajou University School of Medicine, Suwon, Korea. sejoong@ajou.ac.kr
- 2Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2294234
- DOI: http://doi.org/10.4111/kju.2006.47.6.607
Abstract
-
PURPOSE: We investigated the relationship between the expression of caveolin-1, using a tissue microarray (TMA), and the prognosis of patients with renal cell carcinoma (RCC).
MATERIALS AND METHODS
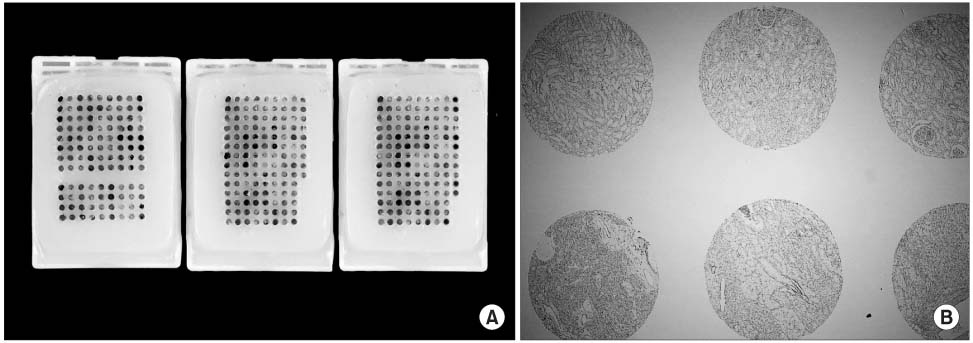
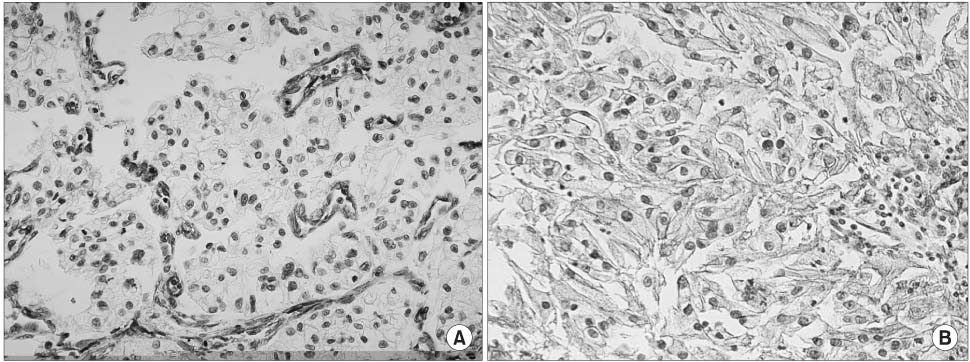
TMA sections of formalin-fixed, paraffin-embedded tissues of RCC from 82 patients, who had undergone radical nephrectomy, were stained immunohistochemically with specific antibodies against caveolin-1. The caveolin-1 immunostaining was semi-quantitatively estimated based on intensity. The expression pattern of caveolin-1 was compared with the clinicopathological variables.
RESULTS
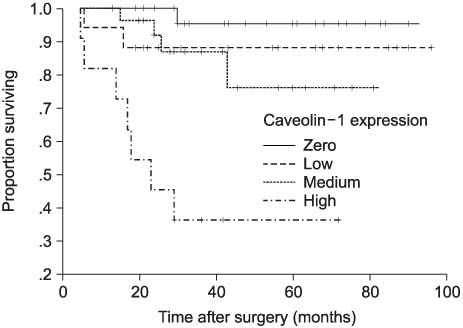
The expression of caveolin-1 was significantly correlated with tumor size (p=0.002), TNM stage (p<0.001), T stage (p=0.001), M stage (p=0.004), grade (p=0.028) and metastasis (p<0.001), and was also significantly higher in clear cell than non-clear cell RCC (p<0.001). The survival of patients with higher caveolin-1 expression was significantly worse than that of patients with lower caveolin-1 expression (p=0.001). Univariate analyses were able to identify all variables, including caveolin-1 expression as significant prognostic factors for cancer-specific survival; multivariate analyses indicated that TNM stage, M stage and grade were independent prognostic factors. Caveolin-1 expression was not an independent factor.
CONCLUSIONS
The increased expression of caveolin-1 is associated with tumor size, stage, grade, metastasis and a worse prognosis in RCC, which suggests that caveolin-1 may be important in the progression of RCC.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Caveolin-1 and Ki-67 Expression as Prognostic Factors in Clear Cell Carcinoma of the Kidney
Byung Hoon Kim, Chun Il Kim, Choal Hee Park
Korean J Urol. 2008;49(2):99-106. doi: 10.4111/kju.2008.49.2.99.
Reference
-
1. Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998. 4:844–847.2. Simon R, Mirlacher M, Sauter G. Tissue microarrays. Biotechniques. 2004. 36:98–105.3. Horvath L, Henshall S. The application of tissue microarrays to cancer research. Pathology. 2001. 33:125–129.4. Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, Gasser TC, et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate. 2003. 55:20–29.5. Nocito A, Bubendorf L, Tinner EM, Süess K, Wagner U, Forster T, et al. Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J Pathol. 2001. 194:349–357.6. Moch H, Schraml P, Bubendorf L, Mirlacher M, Kononen J, Gasser T, et al. High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal cell carcinoma. Am J Pathol. 1999. 154:981–986.7. Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992. 68:673–682.8. Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci USA. 1993. 90:10909–10913.9. Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J Biol Chem. 1998. 273:5419–5422.10. Engelman JA, Zhang X, Galbiati F, Volonte D, Sotgia F, Pestell RG, et al. Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer disease, and muscular dystrophy. Am J Hum Genet. 1998. 63:1578–1587.11. Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, et al. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002. 94:929–933.12. Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, et al. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol. 2001. 159:1635–1643.13. Wikman H, Kettunen E, Seppänen JK, Karjalainen A, Hollmen J, Anttila S, et al. Identification of differentially expressed genes in pulmonary adenocarcinoma by using cDNA array. Oncogene. 2002. 21:5804–5813.14. Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, et al. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002. 160:1745–1754.15. Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, et al. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol. 2001. 158:833–839.16. Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol. 2001. 115:719–724.17. Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002. 87:1140–1144.18. Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998. 4:1873–1880.19. Sanchez-Carbayo M, Socci ND, Charytonowicz E, Lu M, Prystowsky M, Childs G, et al. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002. 62:6973–6980.20. Joo HJ, Oh DK, Kim YS, Lee KB, Kim SJ. Increased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int. 2004. 93:291–296.21. Campbell L, Gumbleton M, Griffiths DF. Caveolin-1 overexpression predicts poor disease-free survival of patients with clinically confined renal cell carcinoma. Br J Cancer. 2003. 89:1909–1913.22. Horiguchi A, Asano T, Asakuma J, Asano T, Sumitomo M, Hayakawa M. Impact of caveolin-1 expression on clinicopathological parameters in renal cell carcinoma. J Urol. 2004. 172:718–722.23. Carrion R, Morgan BE, Tannenbaum M, Salup R, Morgan MB. Caveolin expression in adult renal tumors. Urol Oncol. 2003. 21:191–196.24. Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, et al. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). TNM staging of renal cell carcinoma: Workgroup No. 3. . Cancer. 1997. 80:992–993.25. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982. 6:655–663.26. Rimm DL, Camp RL, Charette LA, Costa J, Olsen DA, Reiss M. Tissue microarray: a new technology for amplification of tissue resources. Cancer J. 2001. 7:24–31.27. Hoos A, Cordon-Cardo C. Tissue microarray profiling of cancer specimens and cell lines: opportunities and limitations. Lab Invest. 2001. 81:1331–1338.28. Merseburger AS, Kuczyk MA, Serth J, Bokemeyer C, Young DY, Sun L, et al. Limitations of tissue microarrays in the evaluation of focal alterations of bcl-2 and p53 in whole mount derived prostate tissues. Oncol Rep. 2003. 10:223–228.29. Pacifico MD, Grover R, Richman P, Daley F, Wilson GD. Validation of tissue microarray for the immunohistochemical profiling of melanoma. Melanoma Res. 2004. 14:39–42.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Expression of Caveolin-1 in Renal Cell Carcinoma
- Correlation between Up-Regulation of Caveolin-1 Gene in Human Rencal Cell Carcinoma and Multi-drug Resistance
- Impact of Caveolin-1 Expression on the Prognosis of Transitional Cell Carcinoma of the Upper Urinary Tract
- Caveolin-1 and Ki-67 Expression as Prognostic Factors in Clear Cell Carcinoma of the Kidney
- The Expression of Caspase 3 and p21 in Renal Cell Carcinoma