J Korean Ophthalmol Soc.
2016 Jun;57(6):1026-1030. 10.3341/jkos.2016.57.6.1026.
A Case of Pigmentary Glaucoma Due to Multidrug-Resistant Tuberculosis Treatment
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University Yangsan Hospital, Yangsan, Korea. jazmin2@naver.com
- 2Department of Ophthalmology, Pusan National University School of Medicine, Busan, Korea.
- 3Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 4Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- KMID: 2290511
- DOI: http://doi.org/10.3341/jkos.2016.57.6.1026
Abstract
- PURPOSE
To report a case of secondary pigmentary glaucoma due to clofazimine treatment for extensive drug-resistant tuberculosis.
CASE SUMMARY
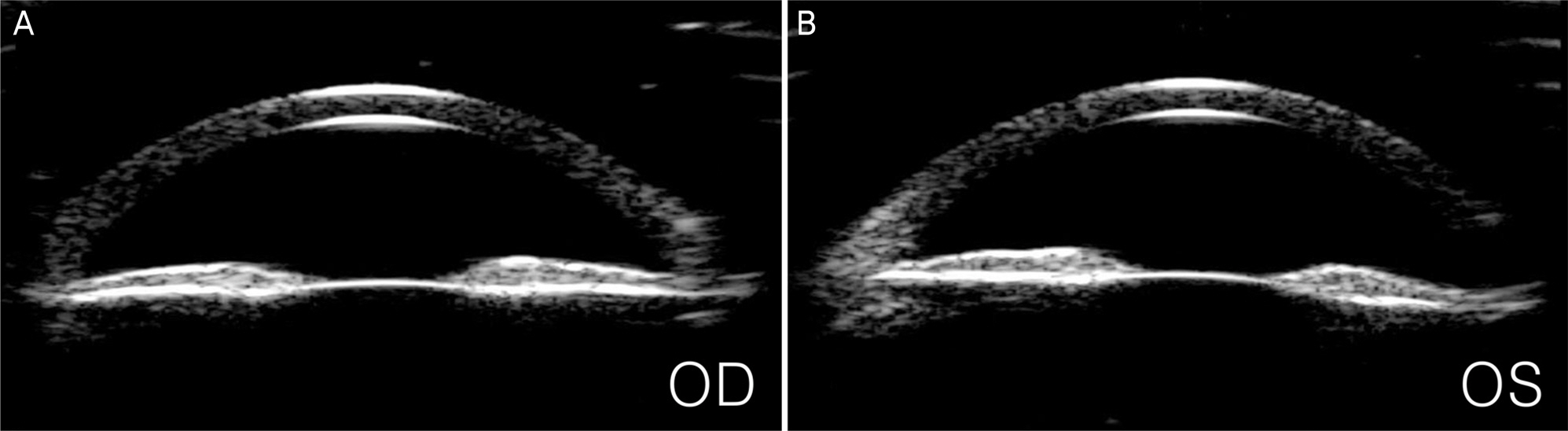
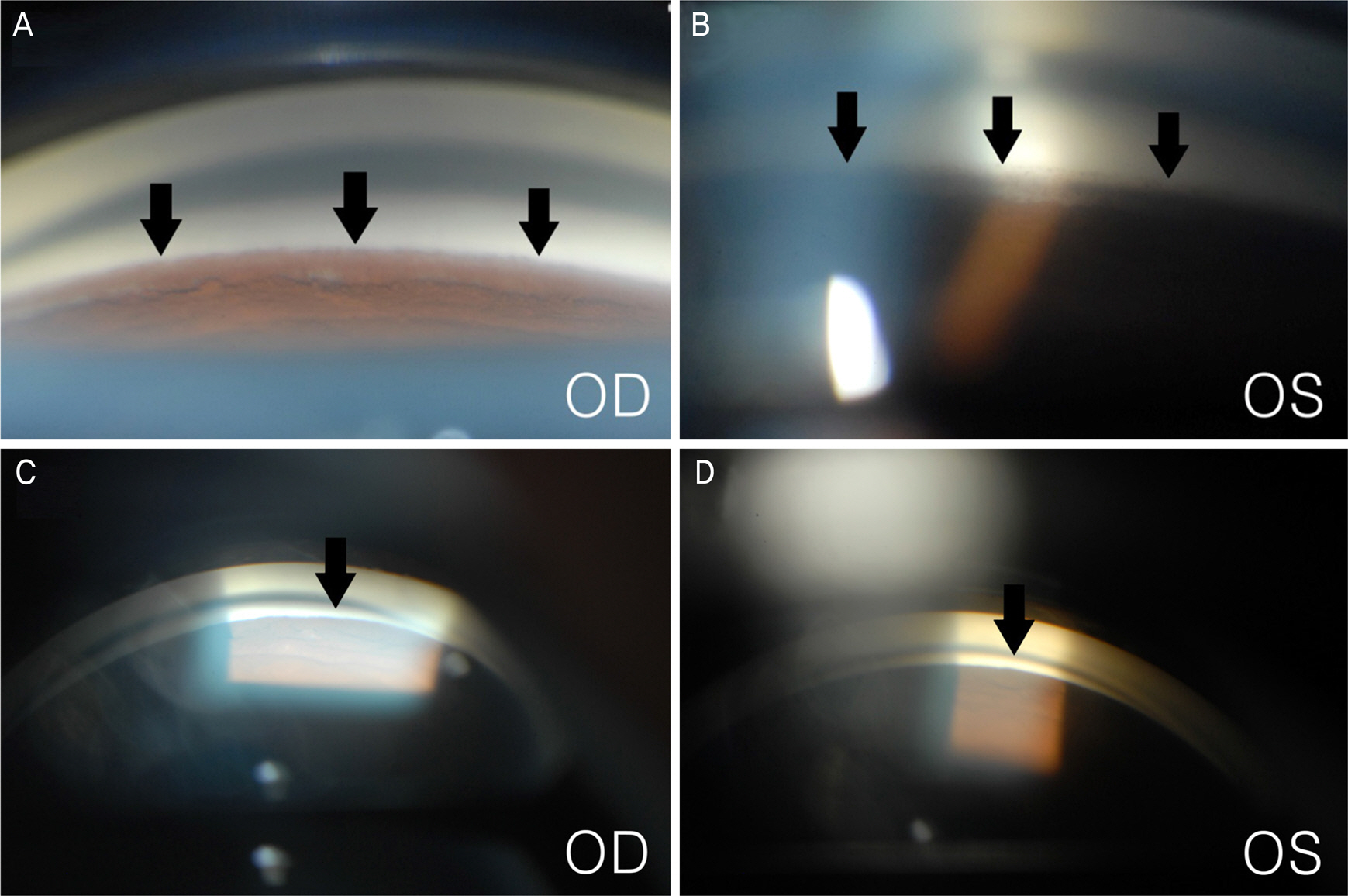
A 23-year-old man presented with blurred vision in both eyes. The patient started to take clofazimine for extensive drug-resistant tuberculosis six months prior, after which his facial skin color changed to a dark-brown. Intraocular pressure (IOP) was 50 mm Hg in the right eye and 48 mm Hg in the left eye. Slit lamp examination revealed corneal edema, opacity, and flare in the anterior chamber in both eyes. A color vision test revealed a mild color defect in both eyes. Visual field (VF) test revealed superior temporal VF loss in the left eye. Gonioscopy revealed open angles with high pigmentation in the trabecular meshwork in both eyes. The patient was diagnosed with pigmentary glaucoma, and maximum tolerated medical therapy was performed. However, the IOP was uncontrolled. Trabeculectomy was performed in both eyes. Postoperative IOP was measured to be 12 mm Hg in both eyes without medication, and visual acuity measured 20/22 in the right eye and 20/17 in the left eye.
CONCLUSIONS
To the best of our knowledge, this report is the first case of clofazimine being a possible cause of pigmentary glaucoma in a patient with extensive drug-resistant tuberculosis.
MeSH Terms
Figure
Reference
-
References
1. Barry VC, Belton JG, Conalty ML, et al. A new series of phena-zines (rimino-compounds) with high antituberculosis activity. Nature. 1957; 179:1013–5.
Article2. Arbiser JL, Moschella SL. Clofazimine: a review of its medical uses and mechanisms of action. J M Acad Dermatol. 1995; 32(2 Pt 1):241–7.
Article3. Ahmad S, Mokaddas E. Current status and future trends in the abdominal and treatment of drug-susceptible and multidrug-resistant tuberculosis. J Infect Public Health. 2014; 7:75–91.4. Morrison NE, Marley GM. Clofazimine binding studies with deox-yribonucleic acid. Int J Lepr Other Mycobact Dis. 1976; 44:475–81.5. Yano T, Kassovska-Bratinova S, Teh JS, et al. Reduction of abdominal by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem. 2011; 286:10276–87.6. Oliva B, O'Neill AJ, Miller K, et al. Anti-staphylococcal activity and mode of action of clofazimine. J Antimicrob Chemother. 2004; 53:435–40.
Article7. Dey T, Brigden G, Cox H, et al. Outcomes of clofazimine for the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2013; 68:284–93.
Article8. Holdiness MR. Clinical pharmacokinetics of clofazimine. A review. Clin Pharmacokinet. 1989; 16:74–85.9. Gopal M, Padayatchi N, Metcalfe JZ, O'Donnell MR. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2013; 17:1001–7.10. Garrelts JC. Clofazimine: a review of its use in leprosy and Mycobacterium avium complex infection. DICP. 1991; 25:525–31.
Article11. Forster DJ, Causey DM, Rao NA. Bull's eye retinopathy and clofazimine. Ann Intern Med. 1992; 116:876–7.
Article12. Niyadurupola N, Broadway DC. Pigment dispersion syndrome and pigmentary glaucoma-a major review. Clin Experiment abdominal. 2008; 36:868–82.13. Farrar SM, Shields MB. Current concepts in pigmentary glaucoma. Surv Ophthalmol. 1993; 37:233–52.
Article14. Campbell DG. Pigmentary dispersion and glaucoma. A new theory. Arch Ophthalmol. 1979; 97:1667–72.15. Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008; 31:127–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multidrug-resistant Tuberculosis Spondylitis: A Case Report
- The survey for clinical course of intractable pulmonary tuberculosis
- Differentiating between Intestinal Tuberculosis and Crohn’s Disease May Be Complicated by Multidrug-resistant Mycobacterium tuberculosis
- Multidrug-resistant Tuberculosis
- Concurrent, Prolonged Use of Bedaquiline and Delamanid for Multidrug-Resistant Tuberculosis