J Breast Cancer.
2013 Sep;16(3):254-265. 10.4048/jbc.2013.16.3.254.
Lymphoma Affecting the Breast: A Pictorial Review of Multimodal Imaging Findings
- Affiliations
-
- 1Department of Radiology, Korea University Ansan Hospital, Ansan, Korea. seoboky@korea.ac.kr
- 2Department of Pathology, Korea University Ansan Hospital, Ansan, Korea.
- 3Department of General Surgery, Korea University Ansan Hospital, Ansan, Korea.
- KMID: 2286376
- DOI: http://doi.org/10.4048/jbc.2013.16.3.254
Abstract
- Hematological malignancies rarely affect the breast, and the majority of those that do are lymphomas. In this review, we describe the clinical aspects and multimodal imaging findings of breast lymphoma. We also illustrate the key clinical and radiological findings that allow it to be distinguished from various other malignant and benign diseases of the breast. Breast lymphoma manifests as a breast mass, a change in the subcutaneous tissue or the skin, or enlargement of the associated lymph node on radiological examination. Radiological findings associated with other breast malignancies, such as calcifications, spiculations, or architectural distortions are extremely rare. Skin and subcutaneous changes frequently accompany T-cell lymphoma. Multimodal breast imaging characteristics may aid in the diagnosis of breast lymphoma.
MeSH Terms
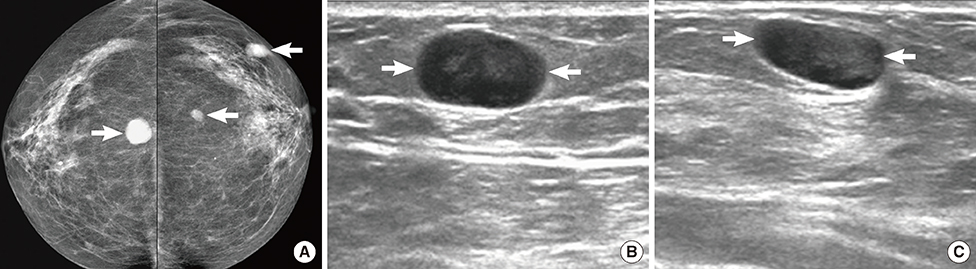
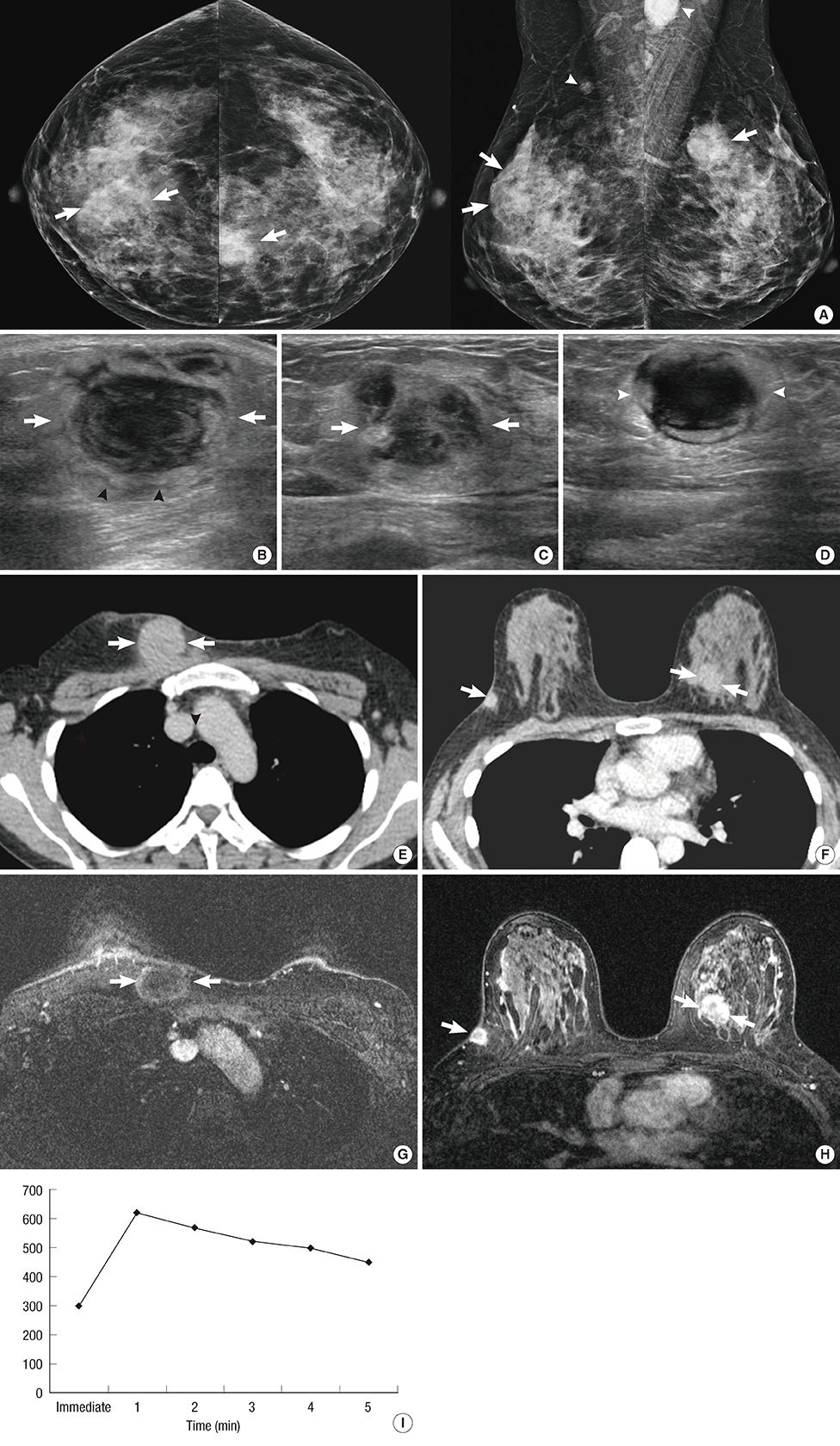
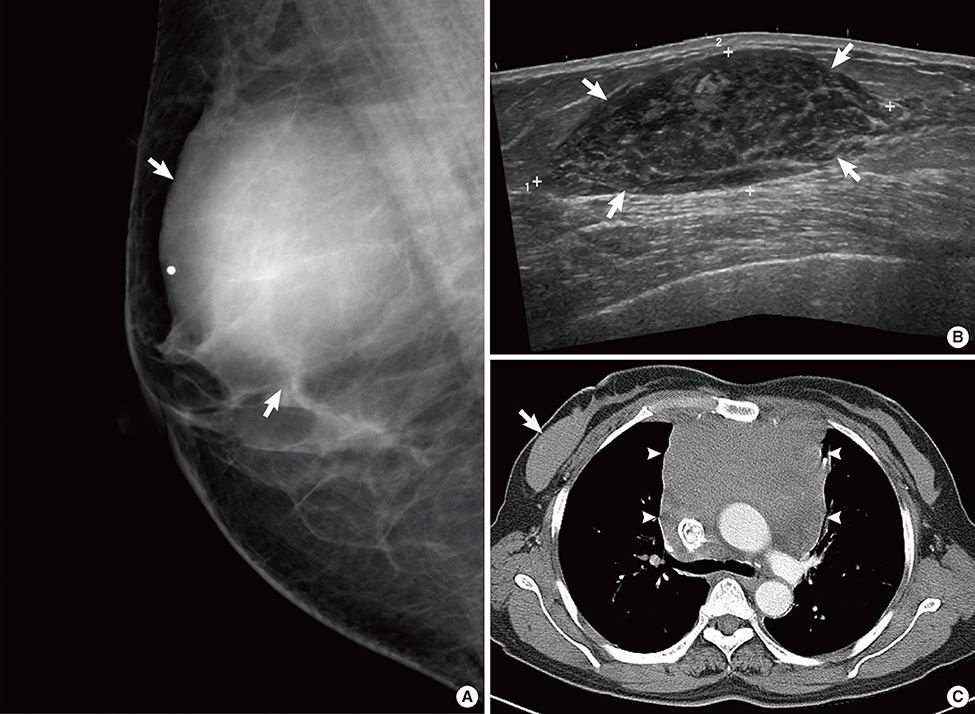
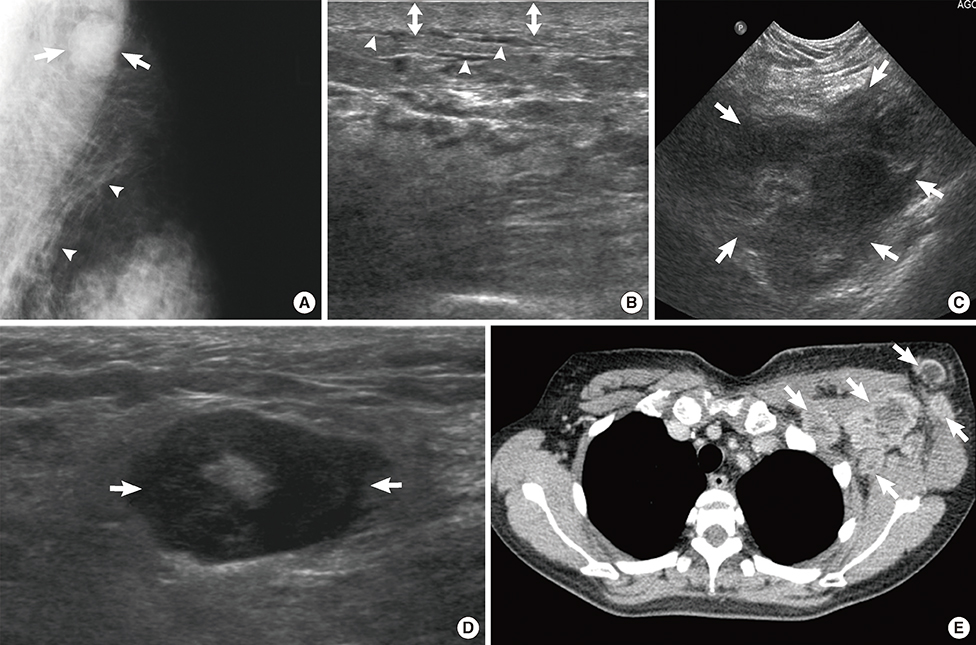
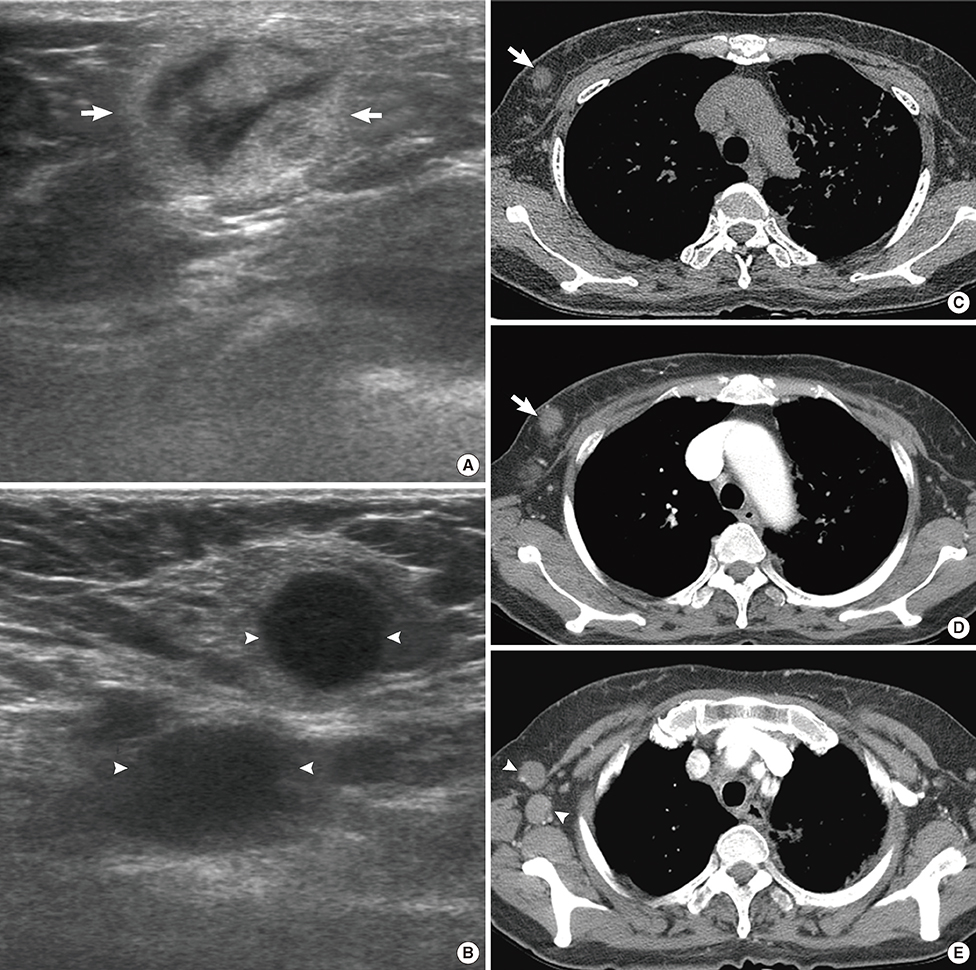
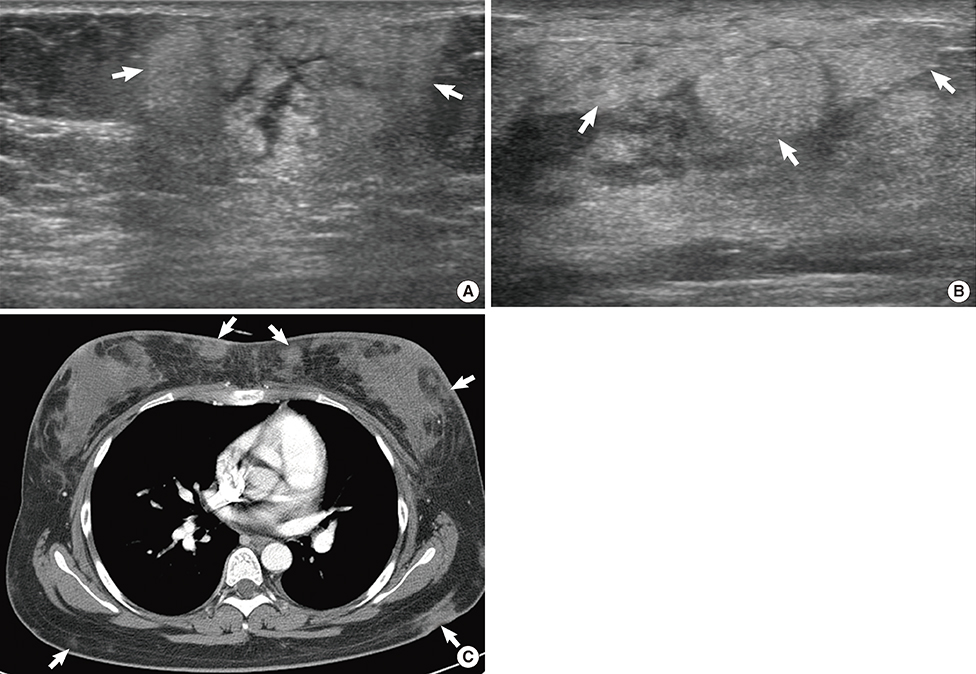
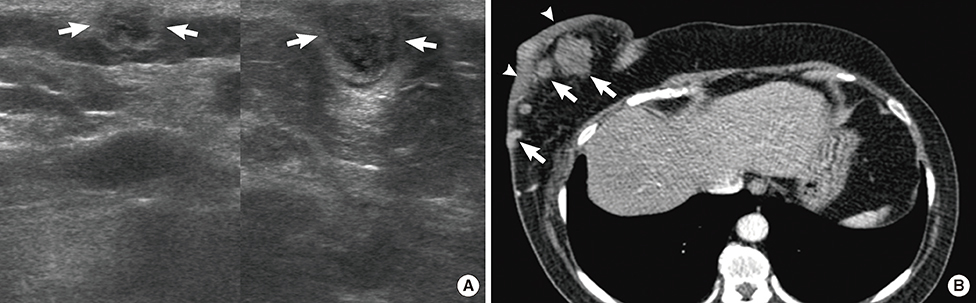
Figure
Reference
-
1. Swerdlow SH. International Agency for Research on Cancer. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC Press;2008.2. Tavassoli FA, Devilee P. International Agency for Research on Cancer. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press;2003. p. 9–112.3. Giardini R, Piccolo C, Rilke F. Primary non-Hodgkin's lymphomas of the female breast. Cancer. 1992; 69:725–735.
Article4. Dao AH, Adkins RB Jr, Glick AD. Malignant lymphoma of the breast: a review of 13 cases. Am Surg. 1992; 58:792–796.5. Brogi E, Harris NL. Lymphomas of the breast: pathology and clinical behavior. Semin Oncol. 1999; 26:357–364.6. Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, et al. ACR Appropriateness Criteria Breast Cancer Screening. Reston: American College of Radiology;2006. p. 217–225.7. Lee WJ, Seo BK, Cho PK, Yie A, Cho KR, Woo OH, et al. The clinical use of low-dose multidetector row computed tomography for breast cancer patients in the prone position. J Breast Cancer. 2010; 13:357–365.
Article8. Yi A, Seo BK, Cho PK, Pisano ED, Lee KY, Je BK, et al. Optimal multidetector row CT parameters for evaluations of the breast: a phantom and specimen study. Acad Radiol. 2010; 17:744–751.
Article9. Seo BK, Pisano ED, Cho KR, Cho PK, Lee JY, Kim SJ. Low-dose multidetector dynamic CT in the breast: preliminary study. Clin Imaging. 2005; 29:172–178.10. American College of Radiology, BI-RADS Committee. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. 4th ed. Reston: American College of Radiology;2003.11. Wiseman C, Liao KT. Primary lymphoma of the breast. Cancer. 1972; 29:1705–1712.
Article12. Surov A, Holzhausen HJ, Wienke A, Schmidt J, Thomssen C, Arnold D, et al. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br J Radiol. 2012; 85:e195–e205.
Article13. Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007; 245:692–702.
Article14. Arber DA, Simpson JF, Weiss LM, Rappaport H. Non-Hodgkin's lymphoma involving the breast. Am J Surg Pathol. 1994; 18:288–295.
Article15. Caon J, Wai ES, Hart J, Alexander C, Truong PT, Sehn LH, et al. Treatment and outcomes of primary breast lymphoma. Clin Breast Cancer. 2012; 12:412–419.
Article16. Avilés A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: results of a controlled clinical trial. Oncology. 2005; 69:256–260.
Article17. Domchek SM, Hecht JL, Fleming MD, Pinkus GS, Canellos GP. Lymphomas of the breast: primary and secondary involvement. Cancer. 2002; 94:6–13.18. Gualco G, Chioato L, Harrington WJ Jr, Weiss LM, Bacchi CE. Primary and secondary T-cell lymphomas of the breast: clinico-pathologic features of 11 cases. Appl Immunohistochem Mol Morphol. 2009; 17:301–306.19. Talwalkar SS, Miranda RN, Valbuena JR, Routbort MJ, Martin AW, Medeiros LJ. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008; 32:1299–1309.20. Portlock C, Vose MJ, Cheson BD. T-cell lymphomas. Lymphoma Research Foundation;2008. Accessed July 24th, 2013. http://www.lymphoma.org/atf/cf/%7B0363CDD6-51B5-427B-BE48-E6AF871ACEC9%7D/T-CELL%20LYMPHOMAS.PDF.21. Uematsu T, Kasami M. 3T-MRI, elastography, digital mammography, and FDG-PET CT findings of subcutaneous panniculitis-like T-cell lymphoma (SPTCL) of the breast. Jpn J Radiol. 2012; 30:766–771.
Article22. Aladily TN, Medeiros LJ, Amin MB, Haideri N, Ye D, Azevedo SJ, et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol. 2012; 36:1000–1008.23. Sahoo S, Rosen PP, Feddersen RM, Viswanatha DS, Clark DA, Chadburn A. Anaplastic large cell lymphoma arising in a silicone breast implant capsule: a case report and review of the literature. Arch Pathol Lab Med. 2003; 127:e115–e118.
Article24. Aguilera NS, Tavassoli FA, Chu WS, Abbondanzo SL. T-cell lymphoma presenting in the breast: a histologic, immunophenotypic and molecular genetic study of four cases. Mod Pathol. 2000; 13:599–605.
Article25. Jewell M, Spear SL, Largent J, Oefelein MG, Adams WP Jr. Anaplastic large T-cell lymphoma and breast implants: a review of the literature. Plast Reconstr Surg. 2011; 128:651–661.26. Smith TJ, Ramsaroop R. Breast implant related anaplastic large cell lymphoma presenting as late onset peri-implant effusion. Breast. 2012; 21:102–104.
Article27. Thompson PA, Lade S, Webster H, Ryan G, Prince HM. Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinico-pathological entity. Haematologica. 2010; 95:1977–1979.
Article28. de Jong D, Vasmel WL, de Boer JP, Verhave G, Barbé E, Casparie MK, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008; 300:2030–2035.
Article29. Friis S, McLaughlin JK, Mellemkjaer L, Kjøller KH, Blot WJ, Boice JD Jr, et al. Breast implants and cancer risk in Denmark. Int J Cancer. 1997; 71:956–958.
Article30. Jeanneret-Sozzi W, Taghian A, Epelbaum R, Poortmans P, Zwahlen D, Amsler B, et al. Primary breast lymphoma: patient profile, outcome and prognostic factors: a Multicentre Rare Cancer Network study. BMC Cancer. 2008; 8:86.
Article31. Yhim HY, Kang HJ, Choi YH, Kim SJ, Kim WS, Chae YS, et al. Clinical outcomes and prognostic factors in patients with breast diffuse large B cell lymphoma: Consortium for Improving Survival of Lymphoma (CISL) study. BMC Cancer. 2010; 10:321.
Article32. Ryan G, Martinelli G, Kuper-Hommel M, Tsang R, Pruneri G, Yuen K, et al. Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2008; 19:233–241.
Article33. Lyou CY, Yang SK, Choe DH, Lee BH, Kim KH. Mammographic and sonographic findings of primary breast lymphoma. Clin Imaging. 2007; 31:234–238.
Article34. Irshad A, Ackerman SJ, Pope TL, Moses CK, Rumboldt T, Panzegrau B. Rare breast lesions: correlation of imaging and histologic features with WHO classification. Radiographics. 2008; 28:1399–1414.
Article35. Yang WT, Metreweli C. Sonography of nonmammary malignancies of the breast. AJR Am J Roentgenol. 1999; 172:343–348.
Article36. Yang WT, Muttarak M, Ho LW. Nonmammary malignancies of the breast: ultrasound, CT, and MRI. Semin Ultrasound CT MR. 2000; 21:375–394.
Article37. Mussurakis S, Carleton PJ, Turnbull LW. MR imaging of primary non-Hodgkin's breast lymphoma: a case report. Acta Radiol. 1997; 38:104–107.38. Naganawa S, Endo T, Aoyama H, Ichihara S. MR imaging of the primary breast lymphoma: a case report. Breast Cancer. 1996; 3:209–213.
Article39. Sy AN, Lam TP, Khoo US. Subcutaneous panniculitislike T-cell lymphoma appearing as a breast mass: a difficult and challenging case appearing at an unusual site. J Ultrasound Med. 2005; 24:1453–1460.
Article40. Lim HJ, Cho KR, Kim I, Hwang KW, Seo BK, Woo OH, et al. Primary peripheral T-cell lymphoma of the breast: radiologic and pathologic findings. J Breast Cancer. 2010; 13:318–322.
Article41. Ko ES, Seol H, Shin JH, Ko EY. Primary anaplastic lymphoma kinase-negative anaplastic large-cell lymphoma of the breast in a male patient. Br J Radiol. 2012; 85:e79–e82.
Article42. Adrada B, Wu Y, Yang W. Hyperechoic lesions of the breast: radiologic-histopathologic correlation. AJR Am J Roentgenol. 2013; 200:W518–W530.
Article43. Heywang-Köbrunner SH, Dershaw DD, Schreer I. Diagnostic Breast Imaging: Mammography, Sonography, Magnetic Resonance Imaging, and Interventional Procedures. 2nd ed. New York: Thieme;2001. p. 236–251. p. 325–338.44. Sun LM, Huang EY, Meng FY, Chang NJ, Chung LM, Liang JA, et al. Primary breast lymphoma clinically mimicking acute mastitis: a case report. Tumori. 2011; 97:233–235.
Article45. Grubstein A, Givon-Madhala O, Morgenstern S, Cohen M. Extranodal primary B-cell non-Hodgkin lymphoma of the breast mimicking acute mastitis. J Clin Ultrasound. 2005; 33:140–142.
Article46. Pinho MC, Souza F, Endo E, Chala LF, Carvalho FM, de Barros N. Nonnecrotizing systemic granulomatous panniculitis involving the breast: imaging correlation of a breast cancer mimicker. AJR Am J Roentgenol. 2007; 188:1573–1576.
Article47. Taboada JL, Stephens TW, Krishnamurthy S, Brandt KR, Whitman GJ. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009; 192:815–825.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imaging Features of the Mesenchymal Tumors of the Breast according to WHO Classification: A Pictorial Essay
- A Rare Case of Male Primary Breast Lymphoma
- Breast Lymphoma: A Report of 2 Cases
- Anomalous Origin of the Coronary Artery from the Pulmonary Artery in Children and Adults: A Pictorial Review of Cardiac Imaging Findings
- MR Spectroscopy and Diffusion Weighted Imaging Findings of Primary Non-Hodgkin Lymphoma of the Breast: Two Case Reports