Ann Dermatol.
2010 May;22(2):156-162. 10.5021/ad.2010.22.2.156.
An Open, Randomized, Comparative Clinical and Histological Study of Imiquimod 5% Cream Versus 10% Potassium Hydroxide Solution in the Treatment of Molluscum Contagiosum
- Affiliations
-
- 1Department of Dermatology, Yansan Pusan National University Hospital, School of Medicine, Pusan National University, Yangsan, Korea.
- 2Department of Family Medicine, Yansan Pusan National University Hospital, School of Medicine, Pusan National University, Yangsan, Korea. dwjeong75@hanmail.net
- 3Department of Orthopaedics, School of Medicine, Dong-a University Medical Center, Busan, Korea.
- KMID: 2265385
- DOI: http://doi.org/10.5021/ad.2010.22.2.156
Abstract
- BACKGROUND
Although molluscum contagiosum (MC) resolves spontaneously, there are several reasons to treat this dermatological disorder.
OBJECTIVE
To evaluate the safety and efficacy of 5% imiquimod cream versus 10% potassium hydroxide (KOH) solution in treating MC, and to propose the mechanism of cure by observing the histological findings.
METHODS
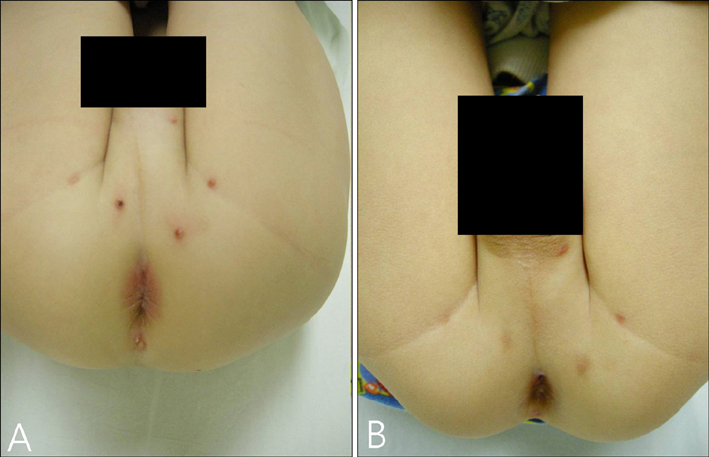
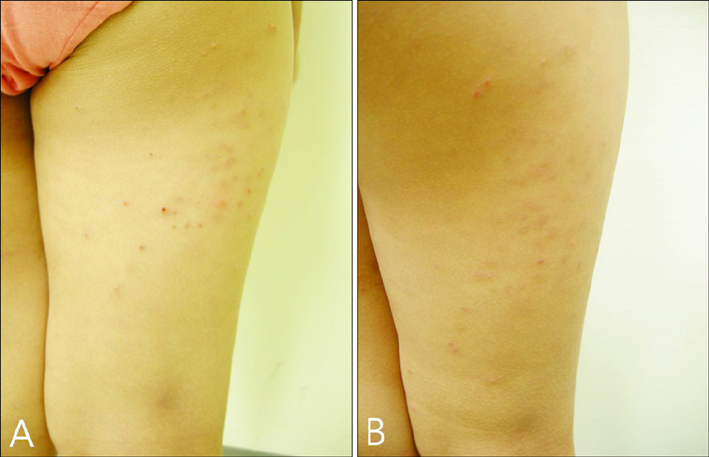
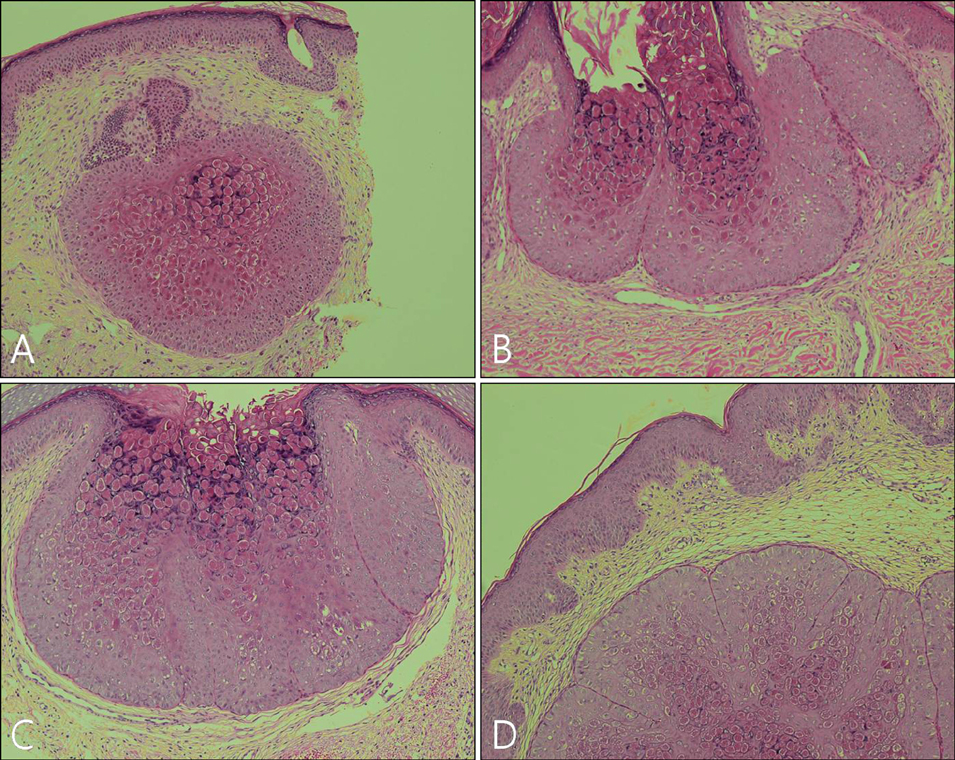
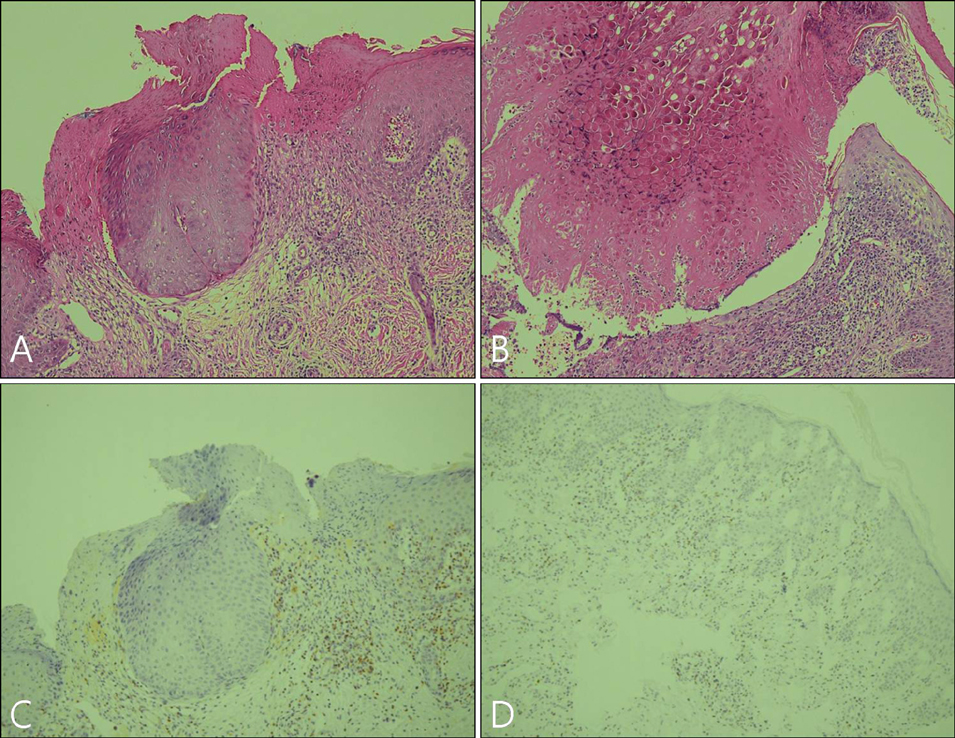
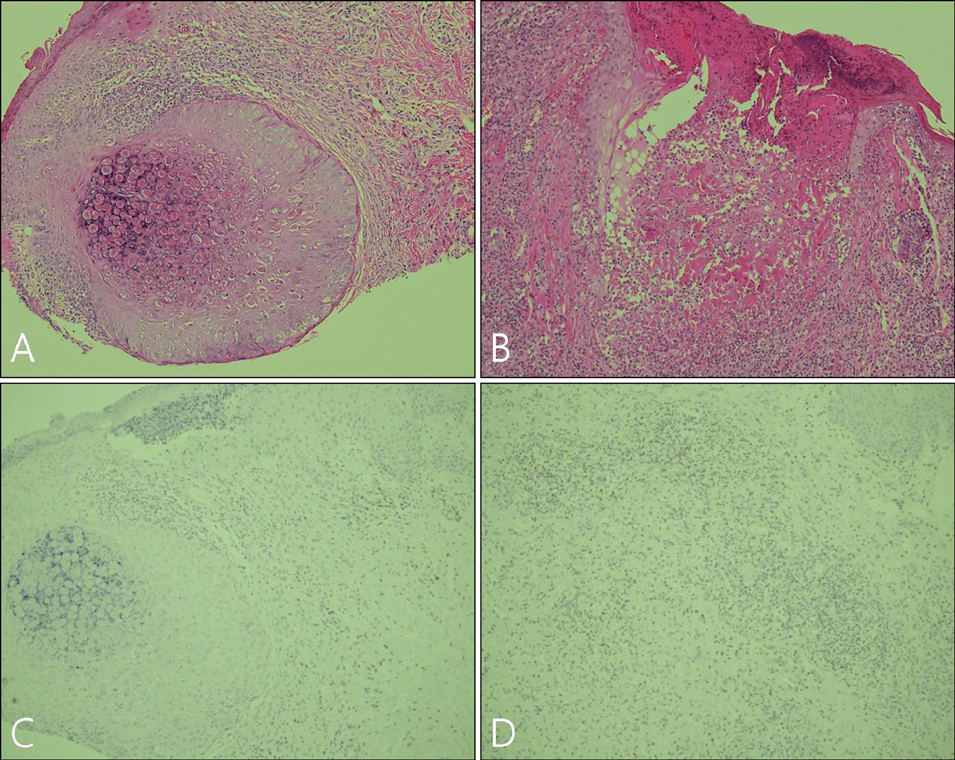
Imiquimod or KOH were applied by the patient or a parent 3 days per week until all lesions cleared. The number of MC lesions was counted and side effects were evaluated at 5 points during the treatment (the initial visit, week 2, week 4, week 8, and week 12). Histological changes were compared between 2 patients of each group, before and after the 2 weeks of application. RESULTS: In both group, the mean lesion counts decreased all through to week 12, and the reduction in number of lesions were statistically significant in both groups (p <0.005). Over 40% of each group developed local side effects, and no systemic side effects were noted in either group. Before treatment, histological findings showed little or no dermal infiltrates. After treatment, specimens showed dense lymphocytic infiltrates, especially T cells, around the lesions which had resolved.
CONCLUSION
Both 10% KOH solution and 5% imiquimod cream are effective and safe treatment of MC.
MeSH Terms
Figure
Reference
-
1. Grayson W, Calonge E, McKee PH. McKee PH, Calonje E, Granter SR, editors. Infectious diseases of the skin. Pathology of the skin: with clinical correlations. 2005. 3rd ed. Philadelphia: Elsevier Mosby;838–992.2. Scheinfeld N. Treatment of molluscum contagiosum: a brief review and discussion of a case successfully treated with adapelene. Dermatol Online J. 2007. 13:15.
Article3. Callen JP. Callen JP, Horn TH, Mancini AJ, editors. Immunomodulators. Dermatology. 2008. 2nd ed. Philadelphia: Mosby Elsevier;1974–1989.4. Romiti R, Ribeiro AP, Grinblat BM, Rivitti EA, Romiti N. Treatment of molluscum contagiosum with potassium hydroxide: a clinical approach in 35 children. Pediatr Dermatol. 1999. 16:228–231.
Article5. Gould D. An overview of molluscum contagiosum: a viral skin condition. Nurs Stand. 2008. 22:45–48.
Article6. Roberts S. Warts and molluscum: an impractical guide. Arch Dis Child Educ Pract Ed. 2007. 92:199–200.7. Simonart T, De Maertelaer V. Curettage treatment for molluscum contagiosum: a follow-up survey study. Br J Dermatol. 2008. 159:1144–1147.
Article8. Bayerl C, Feller G, Goerdt S. Experience in treating molluscum contagiosum in children with imiquimod 5% cream. Br J Dermatol. 2003. 149:Suppl 66. 25–29.
Article9. Cathcart S, Coloe J, Morrell DS. Parental satisfaction, efficacy, and adverse events in 54 patients treated with cantharidin for molluscum contagiosum infection. Clin Pediatr (Phila). 2009. 48:161–165.
Article10. Barba AR, Kapoor S, Berman B. An open label safety study of topical imiquimod 5% cream in the treatment of Molluscum contagiosum in children. Dermatol Online J. 2001. 7:20.11. Theos AU, Cummins R, Silverberg NB, Paller AS. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double-blind, randomized pilot trial. Cutis. 2004. 74:134–138. 141–142.12. Romiti R, Ribeiro AP, Romiti N. Evaluation of the effectiveness of 5% potassium hydroxide for the treatment of molluscum contagiosum. Pediatr Dermatol. 2000. 17:495.
Article13. Metkar A, Pande S, Khopkar U. An open, nonrandomized, comparative study of imiquimod 5% cream versus 10% potassium hydroxide solution in the treatment of molluscum contagiosum. Indian J Dermatol Venereol Leprol. 2008. 74:614–618.
Article14. Arany I, Tyring SK. Activation of local cell-mediated immunity in interferon-responsive patients with human papillomavirus-associated lesions. J Interferon Cytokine Res. 1996. 16:453–460.
Article15. Schneider-Schaulies S, Dittmer U. Silencing T cells or T-cell silencing: concepts in virus-induced immunosuppression. J Gen Virol. 2006. 87:1423–1438.
Article16. Wainberg MA, Mills EL. Mechanisms of virus-induced immune suppression. Can Med Assoc J. 1985. 132:1261–1267.