Ann Dermatol.
2013 Nov;25(4):504-505. 10.5021/ad.2013.25.4.504.
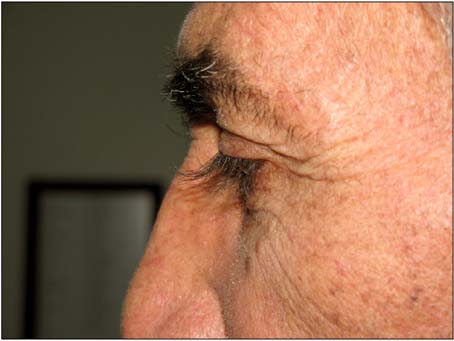
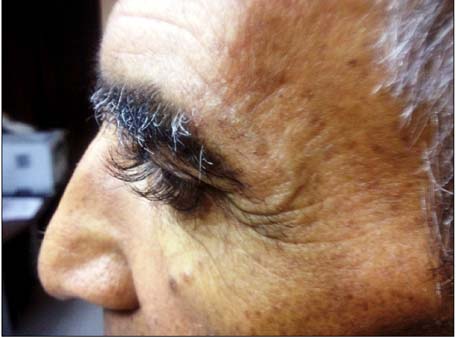
Cetuximab Related Eyelash Elongations for Patients with Metastatic Rectum Carcinoma: Metabolic Complete Response
- Affiliations
-
- 1Department of Medical Oncology, Ankara University School of Medicine, Ankara, Turkey. yukselurun@gmail.com
- KMID: 2265075
- DOI: http://doi.org/10.5021/ad.2013.25.4.504
Abstract
- No abstract available.
Figure
Reference
-
1. Winder T, Lenz HJ. Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev. 2010; 36:550–556.
Article2. De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008; 19:508–515.
Article3. Ocvirk J, Cencelj S. Management of cutaneous side-effects of cetuximab therapy in patients with metastatic colorectal cancer. J Eur Acad Dermatol Venereol. 2010; 24:453–459.
Article4. Saif MW, Kim R. Incidence and management of cutaneous toxicities associated with cetuximab. Expert Opin Drug Saf. 2007; 6:175–182.
Article5. Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005; 16:1425–1433.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retrospective Analysis of Patients Treated with Cetuximab plus FOLFIRI for Previous Irinotecan-combined Chemotherapy in Metastatic Colorectal Cancer
- Skin Metastasis of Neuroendocrine Carcinoma Arising in the Rectum
- A Case of Paronychia Occurring after Injection with Cetuximab (Erbitux(R), IMC-C225)
- Targeted Molecular Therapy in a Murine Model of Oral Squamous Cell Carcinoma with an Epidermal Growth Factor Receptor Inhibitor
- A Case of Metastatic Adenocarcinoma on the Scalp from the Rectum