Ann Dermatol.
2015 Apr;27(2):133-141. 10.5021/ad.2015.27.2.133.
Periadnexal Mucin as an Additional Histopathologic Feature of Chronic Eczematous Dermatitis
- Affiliations
-
- 1Department of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea. ahnsk@yonsei.ac.kr
- 2Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2264801
- DOI: http://doi.org/10.5021/ad.2015.27.2.133
Abstract
- BACKGROUND
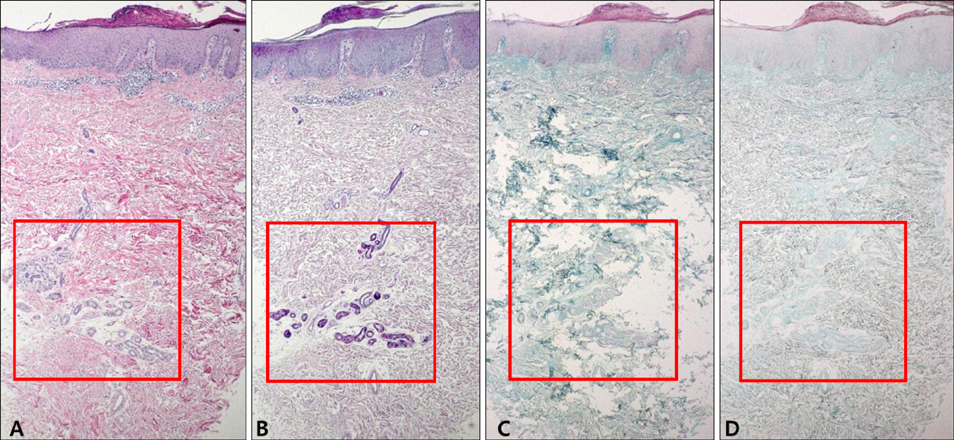
Cutaneous mucinoses are a heterogeneous group of disorders characterized by an abnormal amount of mucin in the skin. However, the pathomechanism of an excessive mucin deposition in the skin is still unknown. Eczematous dermatitis is sub-classified histologically into acute, subacute, and chronic variants. The characteristic histopathologic findings for chronic eczema are variable. However, periadnexal mucin deposition is not known as a feature of chronic eczema.
OBJECTIVE
To evaluate the presence of periadnexal mucin deposition in chronic eczematous dermatitis.
METHODS
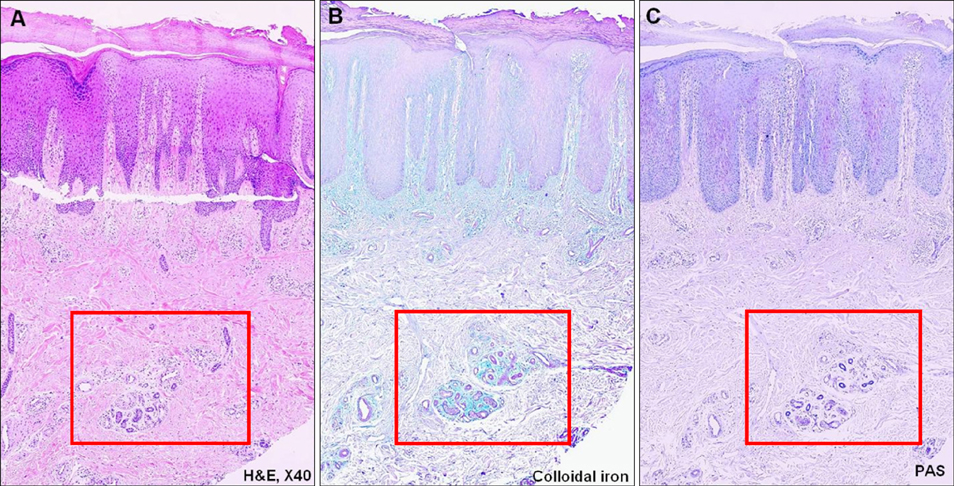
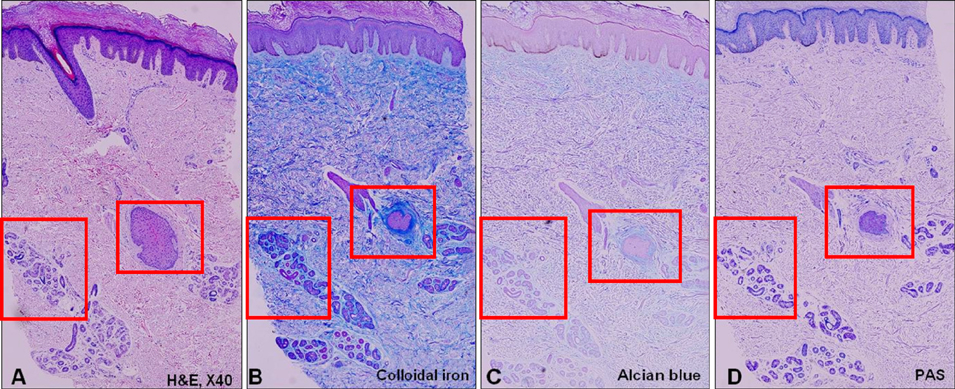
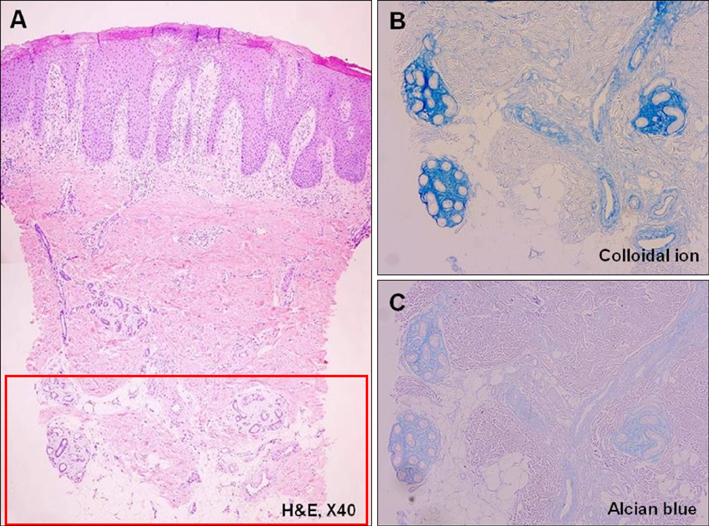
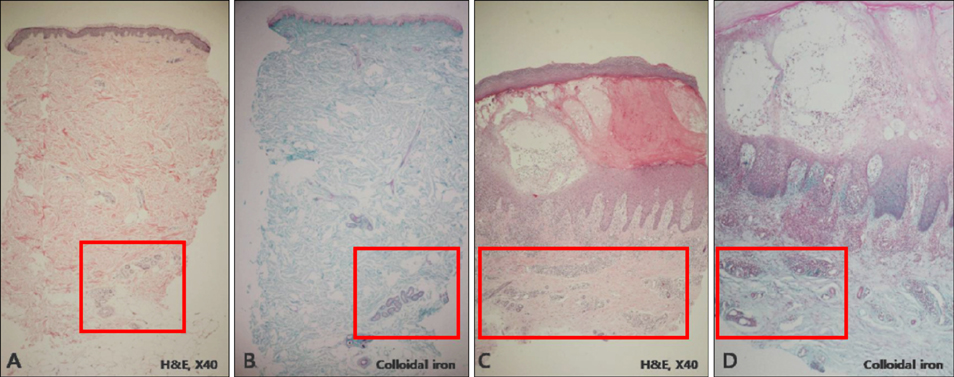
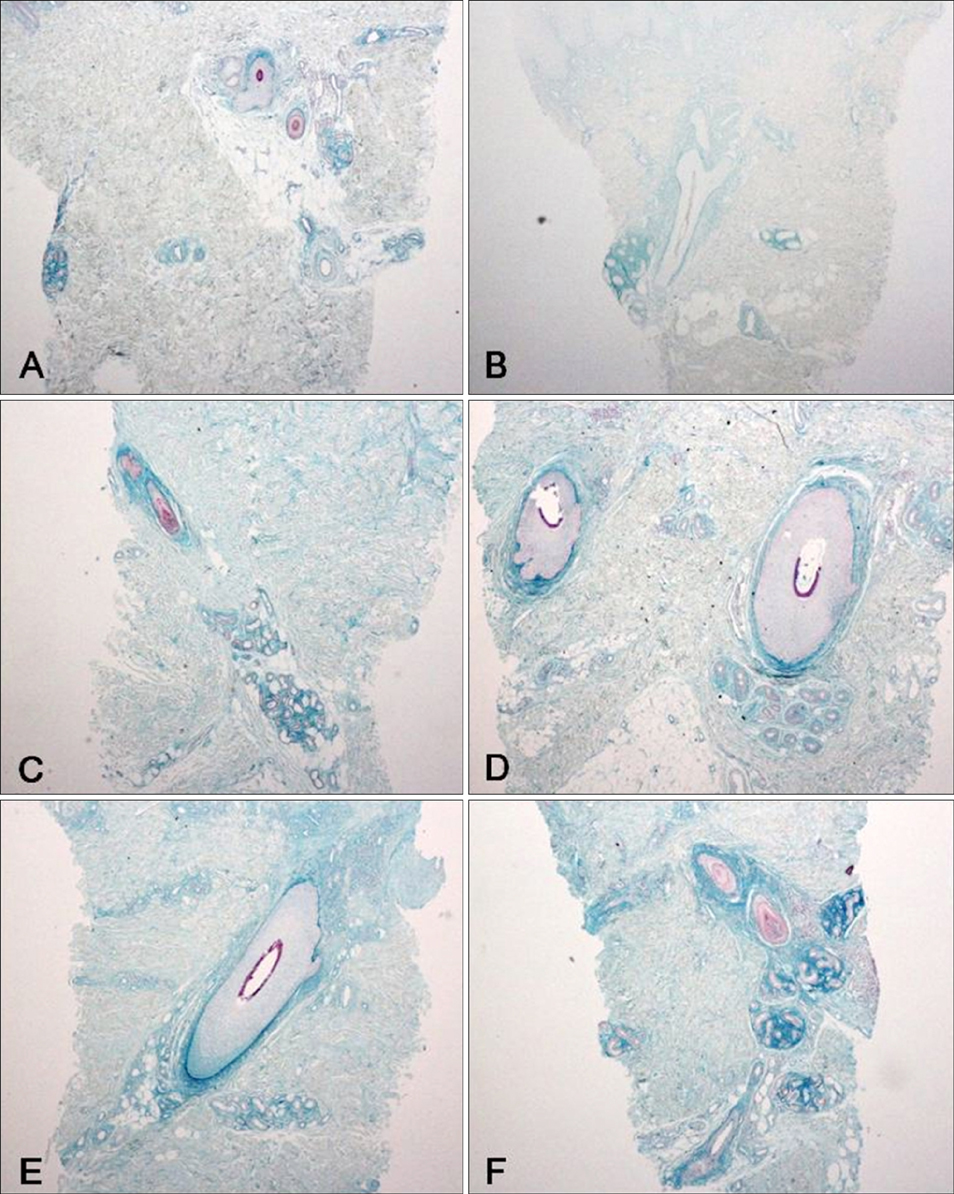
We analyzed the skin biopsy specimens from 36 patients who were pathologically diagnosed with chronic eczematous dermatitis. Alcian blue, colloidal iron, and periodic acid-Schiff stains were used to evaluate the mucin deposition in histologic sections. Two dermatologists and two dermatopathologists evaluated the degree of mucin deposition using a 4-point scale.
RESULTS
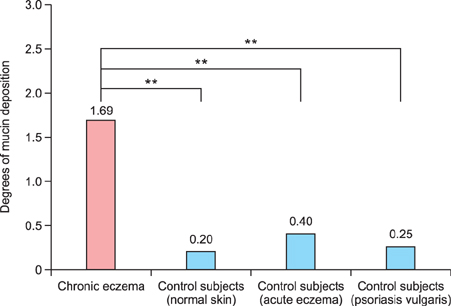
Various amounts of mucin deposition were observed in the periadnexal area of patients who were diagnosed with chronic eczema. Mucin deposition was more visible after staining with mucin-specific stains. Evaluation of the staining analysis scores revealed that the staining intensities were significantly higher in patients with chronic eczema than age- and site-matched controls (normal, acute to subacute eczema, and psoriasis vulgaris).
CONCLUSION
Periadnexal mucin (secondary mucinoses) may be an additional finding of chronic eczematous dermatitis.
Keyword
MeSH Terms
Figure
Reference
-
1. Wintroub B, Arndt K, Robinson J, Leboit P. The cutaneous mucinosis. In : Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, editors. Cutaneous medicine and surgery: an integrated program in dermatology. Philadelphia: WB Saunders Co.;1996. p. 1832–1840.2. Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001; 23:257–267.3. Pandya AG, Sontheimer RD, Cockerell CJ, Takashima A, Piepkorn M. Papulonodular mucinosis associated with systemic lupus erythematosus: possible mechanisms of increased glycosaminoglycan accumulation. J Am Acad Dermatol. 1995; 32:199–205.
Article4. Wu H, Brandling-Bennett HA, Harrist TJ. Noninfectious vesiculobullous and vesiculopustular diseases. In : Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's histopathology of the skin. Philadelphia: Lippincott Williams & Wilkins;2009. p. 237.5. Rebora A, Rongioletti F. Mucinosis. In : Bolognia JL, Jorrizo JL, Rapini RR, editors. Dermatology. Mosby: Elsevier Limited;2003. p. 647.6. Edward M, Fitzgerald L, Thind C, Leman J, Burden AD. Cutaneous mucinosis associated with dermatomyositis and nephrogenic fibrosing dermopathy: fibroblast hyaluronan synthesis and the effect of patient serum. Br J Dermatol. 2007; 156:473–479.
Article7. Harper RA, Rispler J. Lichen myxedematosus serum stimulates human skin fibroblast proliferation. Science. 1978; 199:545–547.
Article8. Cheung HS, Nicoloff JT, Kamiel MB, Spolter L, Nimni ME. Stimulation of fibroblast biosynthetic activity by serum of patients with pretibial myxedema. J Invest Dermatol. 1978; 71:12–17.
Article9. Duncan MR, Berman B. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factoralpha and beta. J Invest Dermatol. 1989; 92:699–706.
Article10. Falanga V, Tiegs SL, Alstadt SP, Roberts AB, Sporn MB. Transforming growth factor-beta: selective increase in glycosaminoglycan synthesis by cultures of fibroblasts from patients with progressive systemic sclerosis. J Invest Dermatol. 1987; 89:100–104.
Article11. Vakirlis E, Lazaridou E, Tzellos TG, Gerou S, Chatzidimitriou D, Ioannides D. Investigation of cytokine levels and their association with SCORAD index in adults with acute atopic dermatitis. J Eur Acad Dermatol Venereol. 2011; 25:409–416.
Article12. Villagomez MT, Bae SJ, Ogawa I, Takenaka M, Katayama I. Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br J Dermatol. 2004; 150:910–916.
Article13. Ackermann L, Harvima IT. Mast cells of psoriatic and atopic dermatitis skin are positive for TNF-alpha and their degranulation is associated with expression of ICAM-1 in the epidermis. Arch Dermatol Res. 1998; 290:353–359.
Article14. Junqueira LC, Montes GS, Martins JE, Joazeiro PP. Dermal collagen distribution. A histochemical and ultrastructural study. Histochemistry. 1983; 79:397–403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of chronic actinic dermatitis
- A Case of Chronic Persistent Photosensitivity
- Difference of Proliferating cell nuclear antigen and epidermal growth factor receptor in psoriases and eczematous dermatitis
- Halo Dermatitis Around a Compound Nevus
- Immunohistochemical Study of Pi Class of Glutathione S-Transferase in Psoriasis and Eczematous Dermatitis