J Gynecol Oncol.
2012 Jul;23(3):175-181. 10.3802/jgo.2012.23.3.175.
Combined panel of serum human tissue kallikreins and CA-125 for the detection of epithelial ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynaecology, National University Health System (NUHS), Yong Loo Lin School of Medicine, National University of Singapore, Singapore. obgmac@nus.edu.sg
- 2Biostatistics Unit, National University Health System (NUHS), Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 3Department of Obstetrics and Gynaecology, Adam Malik Hospital, University of North Sumatera, Medan, Indonesia.
- 4Department of Obstetrics & Gynaecology, Sanglah Denpasar Hospital, University of Udayana, Denpasar, Indonesia.
- 5Department of Obstetrics & Gynaecology, Dr Mohammad Hoesin General Hospital, Uiiversity of Sriwijaya, Palembang, Indonesia.
- 6Department of Obstetrics & Gynaecology, Division of Oncology, University of Indonesia, Jakarta, Indonesia.
- 7Department of Obstetrics & Gynaecology, Dr Sardjito Hospital, University Gadja Mada, Yogyakarta, Indonesia.
- 8Tu Du Hospital, Ho Chi Minh, Vietnam.
- KMID: 2245176
- DOI: http://doi.org/10.3802/jgo.2012.23.3.175
Abstract
OBJECTIVE
To determine the predictive accuracy of the combined panels of serum human tissue kallikreins (hKs) and CA-125 for the detection of epithelial ovarian cancer.
METHODS
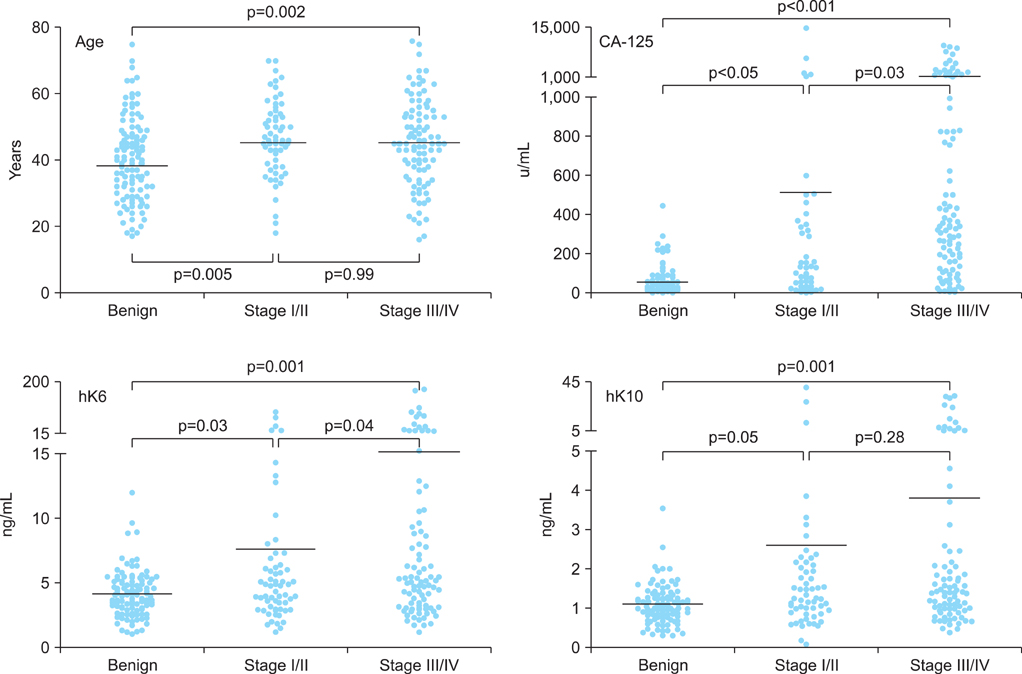
Serum specimens collected from 5 Indonesian centers and 1 Vietnamese center were analyzed for CA-125, hK6, and hK10 levels. A total of 375 specimens from patients presenting with ovarian tumors, which include 156 benign cysts, 172 epithelial ovarian cancers (stage I/II, n=72; stage III/IV, n=100), 36 germ cell tumors and 11 borderline tumors, were included in the study analysis. Receiver operating characteristic analysis were performed to determine the cutoffs for age, CA-125, hK6, and hK10. Sensitivity, specificity, negative, and positive predictive values were determined for various combinations of the biomarkers.
RESULTS
The levels of hK6 and hK10 were significantly elevated in ovarian cancer cases compared to benign cysts. Combination of 3 markers, age/CA-125/hk6 or CA-125/hk6/hk10, showed improved specificity (100%) and positive predictive value (100%) for prediction of ovarian cancer, when compared to the performance of single markers having 80-92% specificity and 74-87% positive predictive value. Four-marker combination, age/CA-125/hK6/hK10 also showed 100% specificity and 100% positive predictive value, although it demonstrated low sensitivity (11.9%) and negative predictive value (52.8%).
CONCLUSION
The combination of human tissue kallikreins and CA-125 showed potential for improving prediction of epithelial ovarian cancer in patients presenting with ovarian tumors.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004. 54:8–29.2. Schink JC. Current initial therapy of stage III and IV ovarian cancer: challenges for managed care. Semin Oncol. 1999. 26:2–7.3. Koh SC, Razvi K, Chan YH, Narasimhan K, Ilancheran A, Low JJ, et al. The association with age, human tissue kallikreins 6 and 10 and hemostatic markers for survival outcome from epithelial ovarian cancer. Arch Gynecol Obstet. 2011. 284:183–190.4. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004. 351:2519–2529.5. Bast RC Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981. 68:1331–1337.6. Rosenthal AN, Jacobs IJ. The role of CA 125 in screening for ovarian cancer. Int J Biol Markers. 1998. 13:216–220.7. Maggino T, Gadducci A. Serum markers as prognostic factors in epithelial ovarian cancer: an overview. Eur J Gynaecol Oncol. 2000. 21:64–69.8. Bast RC Jr, Xu FJ, Yu YH, Barnhill S, Zhang Z, Mills GB. CA 125: the past and the future. Int J Biol Markers. 1998. 13:179–187.9. Rustin GJ, Nelstrop A, Stilwell J, Lambert HE. Savings obtained by CA-125 measurements during therapy for ovarian carcinoma: the North Thames Ovary Group. Eur J Cancer. 1992. 28:79–82.10. Rustin GJ, Nelstrop AE, McClean P, Brady MF, McGuire WP, Hoskins WJ, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996. 14:1545–1551.11. Eisenhauer EA, Vermorken JB, van Glabbeke M. Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: a multivariate analysis of 704 patients. Ann Oncol. 1997. 8:963–968.12. Spentzos D, Levine DA, Ramoni MF, Joseph M, Gu X, Boyd J, et al. Gene expression signature with independent prognostic significance in epithelial ovarian cancer. J Clin Oncol. 2004. 22:4700–4710.13. Kaern J, Aghmesheh M, Nesland JM, Danielsen HE, Sandstad B, Friedlander M, et al. Prognostic factors in ovarian carcinoma stage III patients: can biomarkers improve the prediction of short- and long-term survivors? Int J Gynecol Cancer. 2005. 15:1014–1022.14. Bast RC Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005. 15:Suppl 3. 274–281.15. Badgwell D, Bast RC Jr. Early detection of ovarian cancer. Dis Markers. 2007. 23:397–410.16. Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001. 22:184–204.17. Diamandis EP, Yousef GM. Human tissue kallikreins: a family of new cancer biomarkers. Clin Chem. 2002. 48:1198–1205.18. Diamandis EP, Okui A, Mitsui S, Luo LY, Soosaipillai A, Grass L, et al. Human kallikrein 11: a new biomarker of prostate and ovarian carcinoma. Cancer Res. 2002. 62:295–300.19. Diamandis EP, Scorilas A, Fracchioli S, Van Gramberen M, De Bruijn H, Henrik A, et al. Human kallikrein 6 (hK6): a new potential serum biomarker for diagnosis and prognosis of ovarian carcinoma. J Clin Oncol. 2003. 21:1035–1043.20. Obiezu CV, Diamandis EP. Human tissue kallikrein gene family: applications in cancer. Cancer Lett. 2005. 224:1–22.21. Mills GB, Bast RC Jr, Srivastava S. Future for ovarian cancer screening: novel markers from emerging technologies of transcriptional profiling and proteomics. J Natl Cancer Inst. 2001. 93:1437–1439.22. Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005. 99:267–277.23. Ni X, Zhang W, Huang KC, Wang Y, Ng SK, Mok SC, et al. Characterisation of human kallikrein 6/protease M expression in ovarian cancer. Br J Cancer. 2004. 91:725–731.24. Zheng Y, Katsaros D, Shan SJ, de la Longrais IR, Porpiglia M, Scorilas A, et al. A multiparametric panel for ovarian cancer diagnosis, prognosis, and response to chemotherapy. Clin Cancer Res. 2007. 13:6984–6992.25. Diamandis EP, Yousef GM, Soosaipillai AR, Grass L, Porter A, Little S, et al. Immunofluorometric assay of human kallikrein 6 (zyme/protease M/neurosin) and preliminary clinical applications. Clin Biochem. 2000. 33:369–375.26. Nosov V, Su F, Amneus M, Birrer M, Robins T, Kotlerman J, et al. Validation of serum biomarkers for detection of early-stage ovarian cancer. Am J Obstet Gynecol. 2009. 200:639.e1–639.e5.27. Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005. 102:7677–7682.28. Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996. 2:613–618.29. Borgono CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004. 4:876–890.30. Diamandis EP, Yousef GM, Soosaipillai AR, Bunting P. Human kallikrein 6 (zyme/protease M/neurosin): a new serum biomarker of ovarian carcinoma. Clin Biochem. 2000. 33:579–583.31. Dorn J, Schmitt M, Kates R, Schmalfeldt B, Kiechle M, Scorilas A, et al. Primary tumor levels of human tissue kallikreins affect surgical success and survival in ovarian cancer patients. Clin Cancer Res. 2007. 13:1742–1748.32. Trope C. Prognostic factors in ovarian cancer. Cancer Treat Res. 1998. 95:287–352.33. Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993. 71:1368–1383.34. Duffy MJ. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992. 10:145–155.35. Kramer BS, Gohagan J, Prorok PC, Smart C. A National Cancer Institute sponsored screening trial for prostatic, lung, colorectal, and ovarian cancers. Cancer. 1993. 71:589–593.36. Bjorge T, Engeland A, Sundfor K, Trope CG. Prognosis of 2,800 patients with epithelial ovarian cancer diagnosed during 1975-94 and treated at the Norwegian Radium Hospital. Acta Obstet Gynecol Scand. 1998. 77:777–781.37. Wilson JM, Jungner G. Principles and practice of screening for disease. 1968. Geneva: World Health Organization.38. Chu CS, Rubin SC. Screening for ovarian cancer in the general population. Best Pract Res Clin Obstet Gynaecol. 2006. 20:307–320.39. Rein BJ, Gupta S, Dada R, Safi J, Michener C, Agarwal A. Potential markers for detection and monitoring of ovarian cancer. J Oncol. 2011. 2011:475983.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The clinical value of serum TPS and CA 125 in the diagnosis of epithelial ovarian cancer
- Clinical Relevance of the CA 125 Assay in Monitoring of Epithelial Ovarian Cancer Patients
- Clinical Significance of Serum CA-125 in Korean Females with Ascites
- Correlation between preoperative serum levels of five biomarkers and relationships between these biomarkers and cancer stage in epithelial overian cancer
- Efficacy of Serum CA 125 and Ca 15-3 Levels in Appraently Healthy Women for Ovarian Cancer Screening