Korean J Lab Med.
2006 Oct;26(5):323-338. 10.3343/kjlm.2006.26.5.323.
Establishment of Reference Values for Platelet Activation Markers by Flow Cytometry
- Affiliations
-
- 1Department of Laboratory Medicine, Ajou University School of Medicine, Suwon, Korea. limyoung@ajou.ac.kr
- KMID: 2238843
- DOI: http://doi.org/10.3343/kjlm.2006.26.5.323
Abstract
-
BACKGROUND: This study was purposed to establish reference values for platelet activation markers and leukocyte-platelet aggregates in the evaluation of the platelet function tests using flow cytometry.
METHODS
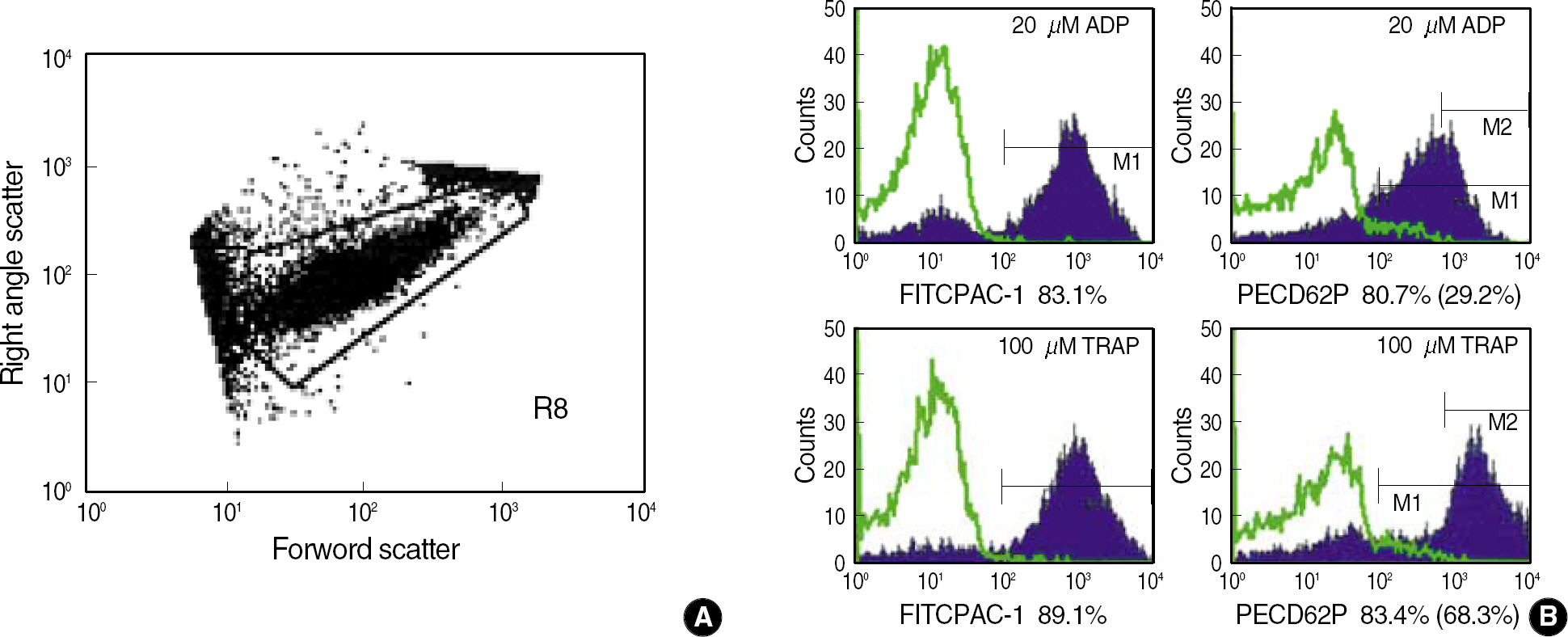
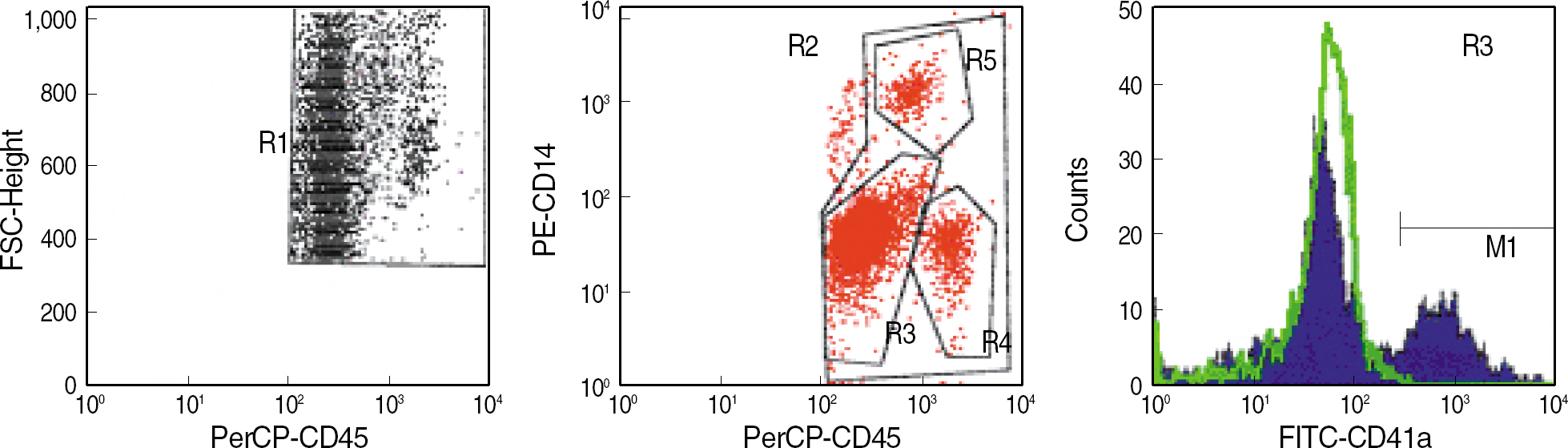
Whole blood samples were obtained from 30 volunteers of healthy adults. Diluted blood samples, either in the resting state or in activated state by the addition of agonist, 20 micrometer ADP or 100 micrometer TRAP, were stained with fluorescent conjugated monoclonal antibody of PAC1 or CD62P. Then, the percentages of expression for each marker were analyzed by flow cytometry. For leukocyte-platelet aggregates, monoclonal antibodies of CD41a, CD14 and CD45 were added simultaneously to undiluted whole blood.
RESULTS
Reference values for the percentages of the expression of PAC1 and CD62P, respectively, were 0.1-12.5% and 0.0-4.7% at the resting state, 65.3-92.4% and 39.0-75.7% with the addition of 20 micrometer ADP, and 68.1-93.1% and 60.5-91.2% with the addition of 100 micrometer TRAP. Reference values for leukocyte-platelet aggregates, granulocyte-platelet aggregates, and lymphocyte-platelets aggregates were 2.8-23.6%, 5.3-34.2%, and 4.9-21.6%, respectively.
CONCLUSIONS
The platelet activation markers at the resting or an activated state with agonists and leukocyte-platelet aggregates could be analyzed using flow cytometry. These reference values should be helpful in interpreting platelet function tests by flow cytometry.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of Nitric Oxide on the Cryopreservation of Platelets
Jae Hyeon Lee, Jeong Tae Kim, Yong Gon Cho
Korean J Lab Med. 2008;28(2):136-143. doi: 10.3343/kjlm.2008.28.2.136.
Reference
-
References
1. Flores NA, Sheridan DJ. The pathophysiological role of platelets during myocardial ischaemia. Cardiovasc Res. 1994; 28:295–302.
Article2. Michelson AD. Flow cytometry: a clinical test of platelet function. Blood. 1996; 87:4925–36.
Article3. Rinder HM, Bonan JL, Rinder CS, Ault KA, Smith BR. Activated and unactivated platelet adhesion to monocytes and neutrophils. Blood. 1991; 78:1760–9.
Article4. Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev. 1993; 7:52–62.5. Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb-IIIa complex during platelet activation. J Biol Chem. 1985; 260:11107–14.
Article6. Xiao Z, Theroux P. Clopidogrel inhibits platelet-leukocyte interactions and thrombin receptor agonist peptide-induced platelet activation in patients with an acute coronary syndrome. J Am Coll Cardiol. 2004; 43:1982–8.
Article7. Yamazaki M, Uchiyama S, Iwata M. Measurement of platelet fibrinogen binding and p-selectin expression by flow cytometry in patients with cerebral infarction. Thromb Res. 2001; 104:197–205.
Article8. Lim YA, Hyun BH. Evaluation of platelet parameters on the ADVIA 120 as the quality indicator for stored platelets. Clin Lab Haematol. 2002; 24:377–84.
Article9. Li N, Goodall AH, Hjemdahl P. A sensitive flow cytometric assay for circulating platelet-leucocyte aggregates. Br J Haematol. 1997; 99:808–16.
Article10. Villmow T, Kemkes-Matthes B, Matzdorff AC. Markers of platelet activation and platelet-leukocyte interaction in patients with myeloproliferative syndromes. Thromb Res. 2002; 108:139–45.
Article11. Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001; 51:639–47.
Article12. Holmes MB, Sobel BE, Howard DB, Schneider DJ. Differences between activation thresholds for platelet P-selectin glycoprotein IIb-IIIa expression and their clinical implications. Thromb Res. 1999; 95:75–82.13. Roshan TM, Normah J, Rehman A, Naing L. Effect of menopause on platelet activation markers determined by flow cytometry. Am J Hematol. 2005; 80:257–61.
Article14. Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, et al. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996; 93:11877–82.
Article15. Hagberg IA, Lyberg T. Evaluation of circulating platelet-leukocyte conjugates: a sensitive flow cytometric assay well suited for clinical studies. Platelets. 2000; 11:151–60.
Article16. Peters MJ, Heyderman RS, Hatch DJ, Klein NJ. Investigation of platelet-neutrophil interactions in whole blood by flow cytometry. J Immunol Methods. 1997; 209:125–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Flow Cytometric Detection of Platelet Activation in Preeclampsia Compared with Uncomplicated Pregnancy
- Changes in Platelet Activation Markers by Leukocyte-Removal Filters
- Platelet Activation During Hemodialysis Measured Through Expression of P-selectin
- Basophil Markers for Identification and Activation in the Indirect Basophil Activation Test by Flow Cytometry for Diagnosis of Autoimmune Urticaria
- Effects of Cytokine on Platelet Activation