J Korean Ophthalmol Soc.
2013 Feb;54(2):199-209. 10.3341/jkos.2013.54.2.199.
Anterior Elevation Changes Following Corneal Crosslinking for Keratoconus
- Affiliations
-
- 1Department of Ophthalmology, Kyung Hee University School of Medicine, Seoul, Korea. khjinmd@khmc.or.kr
- KMID: 2216653
- DOI: http://doi.org/10.3341/jkos.2013.54.2.199
Abstract
- PURPOSE
To report the results according to anterior elevation changes following corneal crosslinking (CXL) treatment for keratoconus.
METHODS
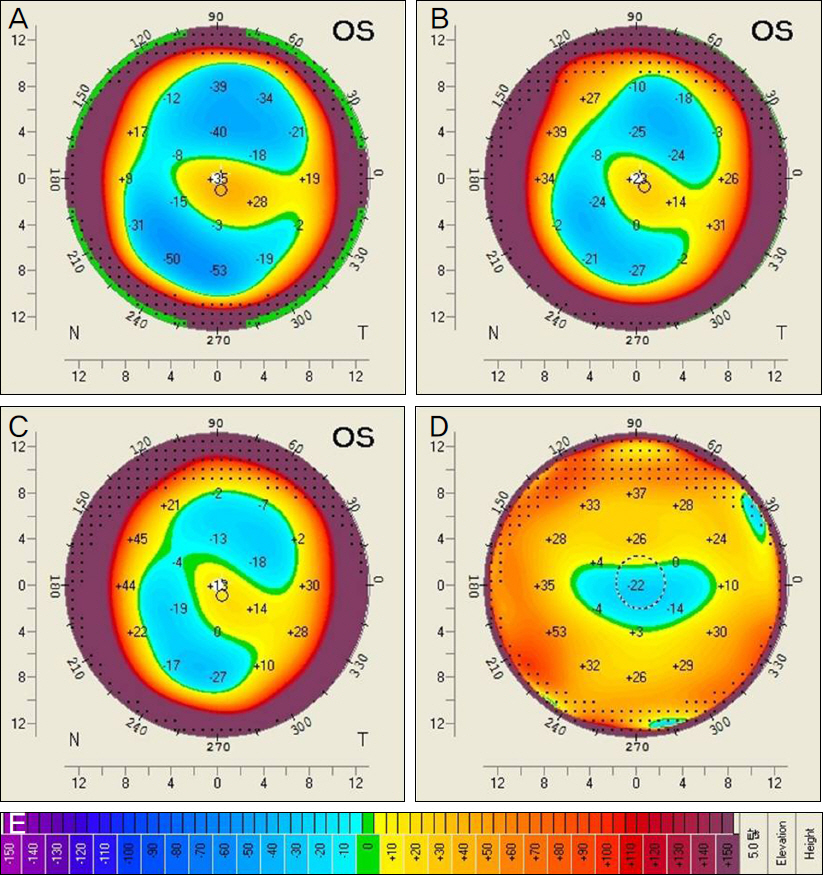
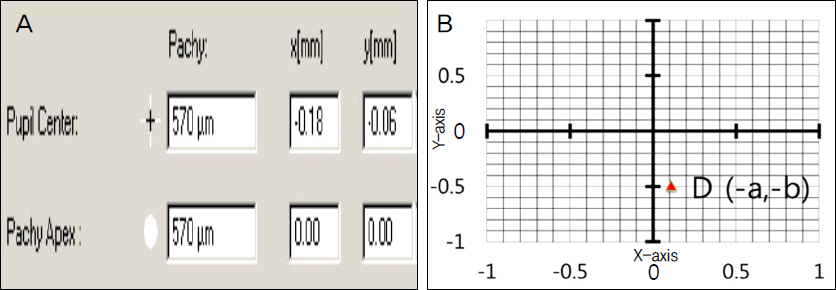
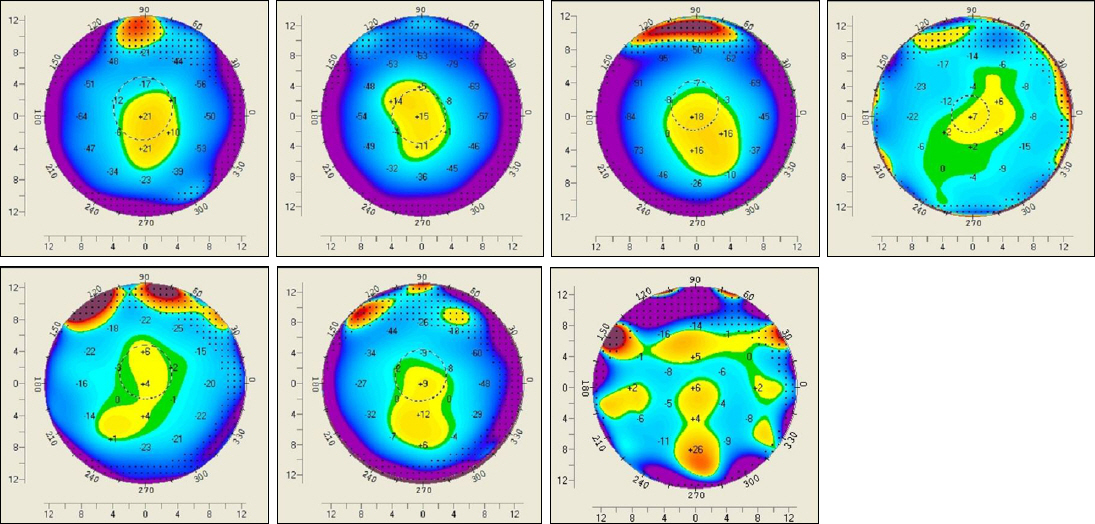
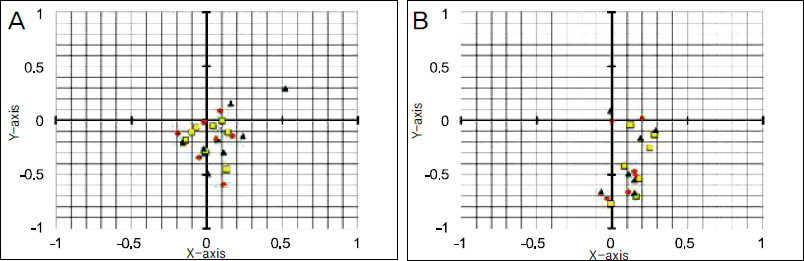
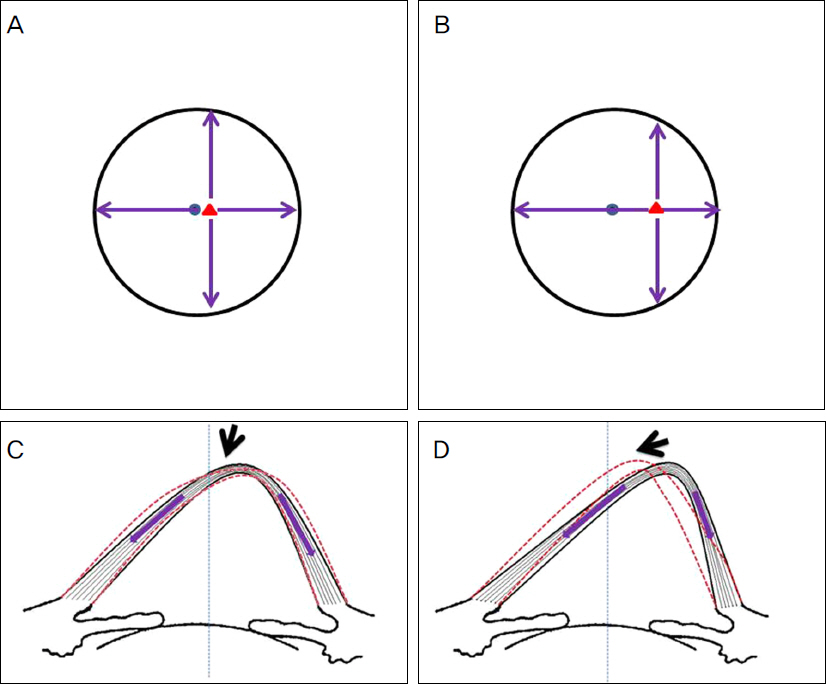
The present retrospective study included 14 patients (15 eyes) with progressive keratoconus who underwent CXL with a follow-up of 12 months. Patients were classified into 2 groups according to pre and postoperative anterior elevation difference maps. On the preoperative anterior elevation map, distances from maximum anterior elevation to pupil center were compared between the 2 groups. The outcome of best correct visual acuity (BCVA), maximum keratometry and parameters of corneal topography were compared between the 2 groups before CXL as well as 6 and 12 months after CXL.
RESULTS
The anterior elevation changes were classified as group 1 (-7.88 +/- 10.53 micrometer) or group 2 (8.71 +/- 5.99 micrometer) (p = 0.001). The preoperative corneal topography of eyes observed in group 1 (0.19 +/- 0.13 mm) had shorter mean distances from maximum anterior elevation to pupil center than eyes in group 2 (0.47 +/- 0.23 mm) (p = 0.018). BCVA (log MAR) improved from 0.68 +/- 0.78 to 0.57 +/- 0.81 (p = 0.115) 12 months after CXL in group 1 and decreased from 0.51 +/- 0.34 to 0.56 +/- 0.38 (p = 0.109) 12 months after CXL in group 2. The maximum keratometry decreased from 63.01 +/- 19.07D to 58.95 +/- 16.32D (p = 0.017) in group 1 and increased from 60.70 +/- 9.46D to 61.29 +/- 7.51D (p = 0.674) in group 2.
CONCLUSIONS
Clinical and optical effects improved postoperatively in group 1, and were stabilized in group 2. The preoperative distance from maximum anterior elevation to pupil center and the anterior elevation changes after CXL were factors in predicting the CXL outcome.
MeSH Terms
Figure
Reference
-
References
1. Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T.Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007; 33:2035–40.
Article2. Wollensak G, Spoerl E, Seiler T.Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135:620–7.
Article3. Tomkins O, Garzozi HJ.Collagen cross-linking: Strengthening the unstable cornea. Clin Ophthalmol. 2008; 2:863–7.
Article4. Hersh PS, Greenstein SA, Fry KL.Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011; 37:149–60.
Article5. Greenstein SA, Fry KL, Hersh PS.Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011; 37:1282–90.
Article6. Caporossi A, Baiocchi S, Mazzotta C, et al. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006; 32:837–45.7. Lee P, Jin KH.Clinical results of riboflavin and ultravioletA-Induced corneal cross-linking for progressive keratoconus in Korean patients. J Korean Ophthalmol Soc. 2011; 52:23–8.
Article8. Maeda N, Klyce SD, Smolek MK.Comparison of methods for detecting keratoconus using videokeratography. Arch Ophthalmol. 1995; 113:870–4.
Article9. Wittig-Silva C, Whiting M, Lamoureux E, et al. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008; 24:S720–5.
Article10. Vinciguerra P, Albé E, Trazza S, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009; 116:369–78.
Article11. Andreassen TT, Simonsen AH, Oxlund H.Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980; 31:435–41.
Article12. Rabinowitz YS.Keratoconus. Surv Ophthalmol. 1998; 42:297–319.
Article13. Daxer A, Fratzl P.Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest Ophthalmol Vis Sci. 1997; 38:121–9.14. Boote C, Dennis S, Huang Y, et al. Lamellar orientation in human cornea in relation to mechanical properties. J Struct Biol. 2005; 149:1–6.
Article15. Polack FM.Contributions of electron microscopy to the study of corneal pathology. Surv Ophthalmol. 1976; 20:375–414.
Article16. Meek KM, Tuft SJ, Huang Y, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005; 46:1948–56.
Article17. Tu KL, Aslanides IM.Orbscan II anterior elevation changes following corneal collagen cross-linking treatment for keratoconus. J Refract Surg. 2009; 25:715–22.
Article18. Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007; 26:385–9.
Article19. Bottós KM, Dreyfuss JL, Regatieri CV, et al. Immunofluorescence confocal microscopy of porcine corneas following collagen cross-linking treatment with riboflavin and ultraviolet A. J Refract Surg. 2008; 24:S715–9.
Article20. Kohlhaas M, Spoerl E, Schilde T, et al. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006; 32:279–83.21. Mazzotta C, Traversi C, Baiocchi S, et al. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol. 2008; 146:527–33.
Article22. Kymionis G, Portaliou D.Corneal crosslinking with riboflavin and UVA for the treatment of keratoconus. J Cataract Refract Surg. 2007; 33:1143–4.
Article23. Patel S, Marshall J, Fitzke FW. 3rd. Model for deriving the optical performance of the myopic eye corrected with an intracorneal ring. J Refract Surg. 1995; 11:248–52.24. Piñero DP, Alio JL, Barraquer RI, Michael R.Corneal bio-mechanical changes after intracorneal ring segment implantation in keratoconus. Cornea. 2012; 31:491–9.
Article25. Koller T, Mrochen M, Seiler T.Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009; 35:1358–62.
Article26. Caporossi A, Mazzotta C, Baiocchi S, Caporossi T.Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy : the Siena eye cross study. Am J Ophthalmol. 2010; 149:585–93.27. Greenstein SA, Shah VP, Fry KL, Hersh PS.Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011; 37:691–700.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corneal Topographic Study Using Orbscan II between Keratoconus and Keratoconus Suspect
- Comparison of Anterior and Posterior Elevation, and Sagittal Curvature between Keratoconus and Normal Cornea
- The Evaluation of Enhanced Ectasia Display Mode in Screening for Keratoconus
- Corneal Crosslinking in Far-Advanced Keratoconus
- Comparison of Anterior, Posterior, and Total Corneal Astigmatism Measured Using a Single Scheimpflug Camera in Healthy and Keratoconus Eyes