J Korean Ophthalmol Soc.
2016 Feb;57(2):208-213. 10.3341/jkos.2016.57.2.208.
The Effect of 3% Diquafosol Tetrasodium on Corneal Wetting Property and Mucin-5AC Concentration in Rabbits
- Affiliations
-
- 1Department of Ophthalmology, Korea University College of Medicine, Seoul, Korea. crisim@korea.ac.kr
- KMID: 2213660
- DOI: http://doi.org/10.3341/jkos.2016.57.2.208
Abstract
- PURPOSE
To evaluate the immediate effects of 3% diquafosol ophthalmic solution on tear MUC5AC concentration and corneal wetting property in rabbit eyes.
METHODS
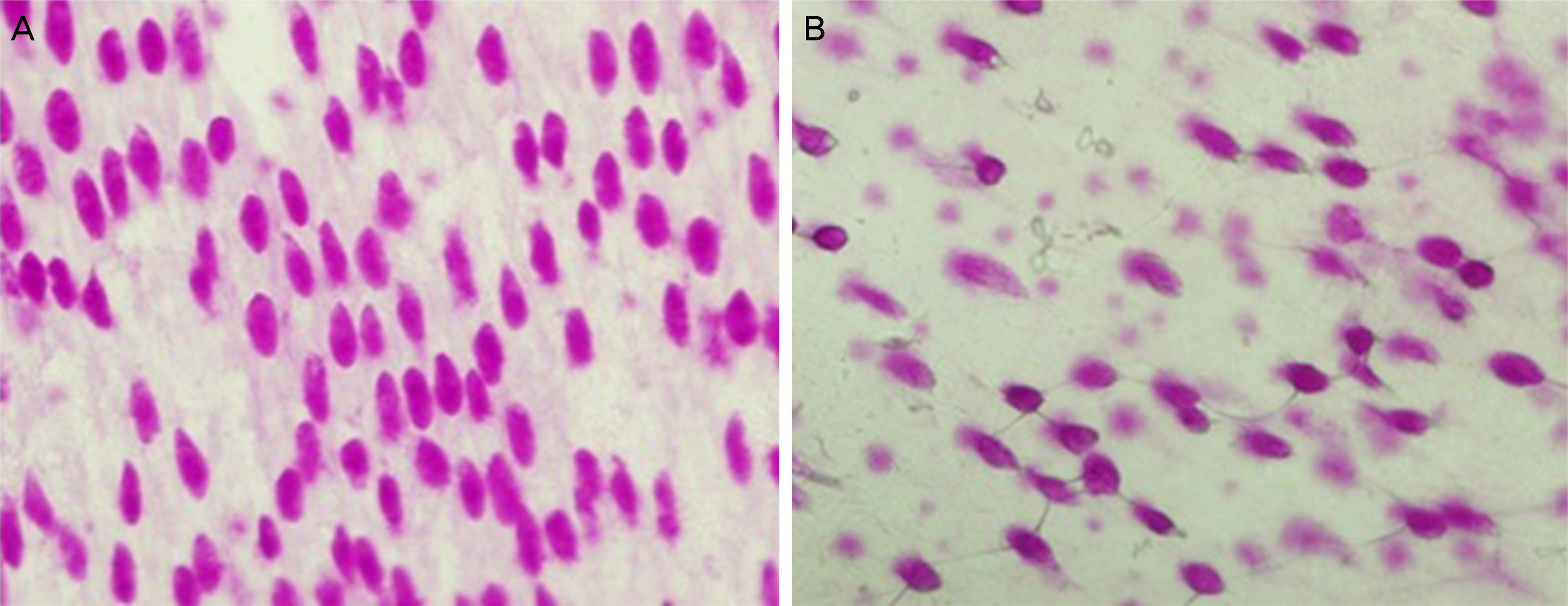
Six New Zealand white rabbits were used in the present study. Fifteen minutes after instilling 50 microL of 3% diquafosol into the right eye of each rabbit and 50 microL of saline into the left eye, corneal wetting property, tear MUC5AC concentrations and the area of periodic acid-Schiff (PAS)-stained conjunctival goblet cells were evaluated under general anesthesia using conjunctival impression cytology. Corneal wetting property was evaluated by measuring the duration from when the image of the microscopic light beam was clear on the corneal surface immediately after blinking to when the image began to blur.
RESULTS
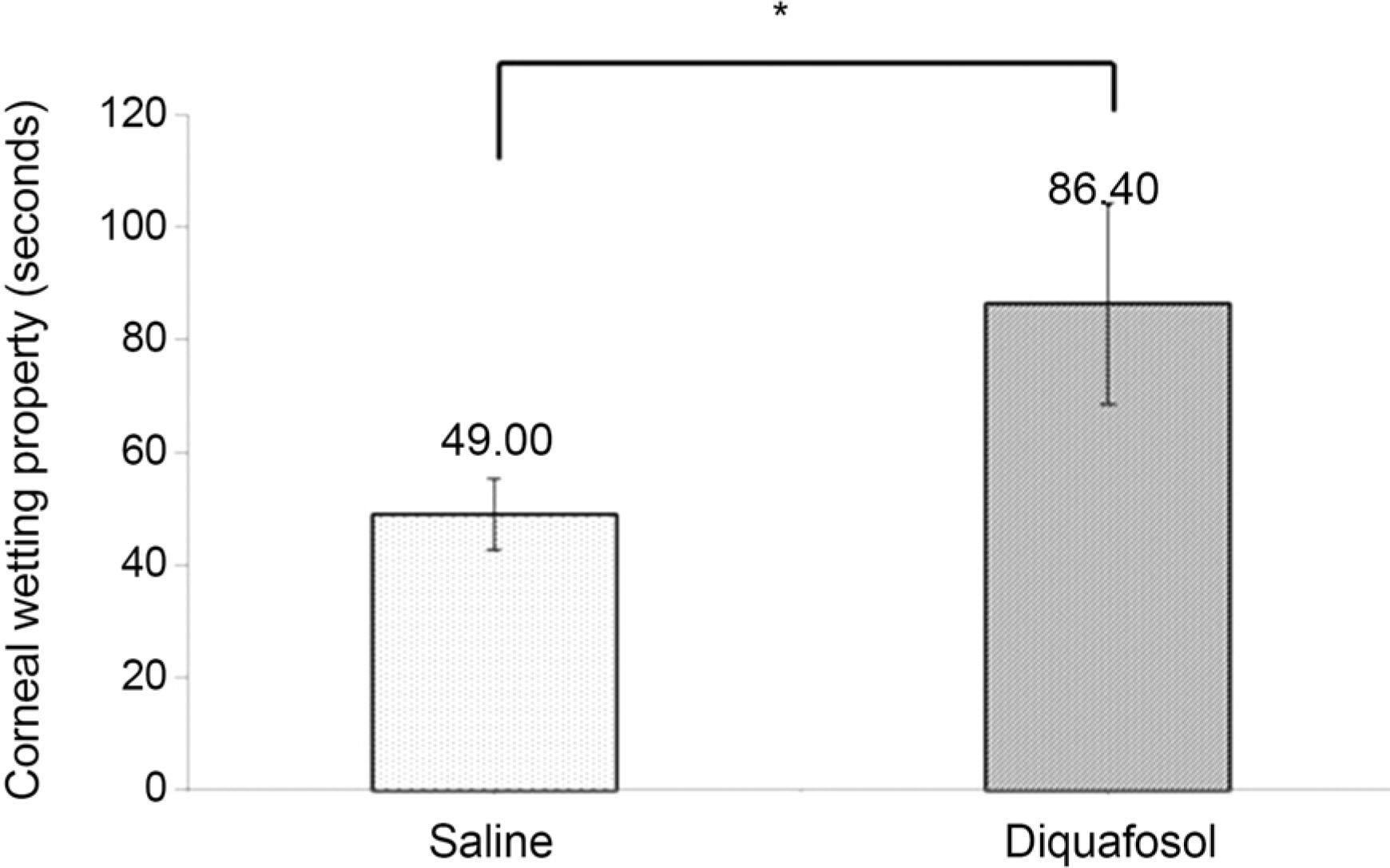
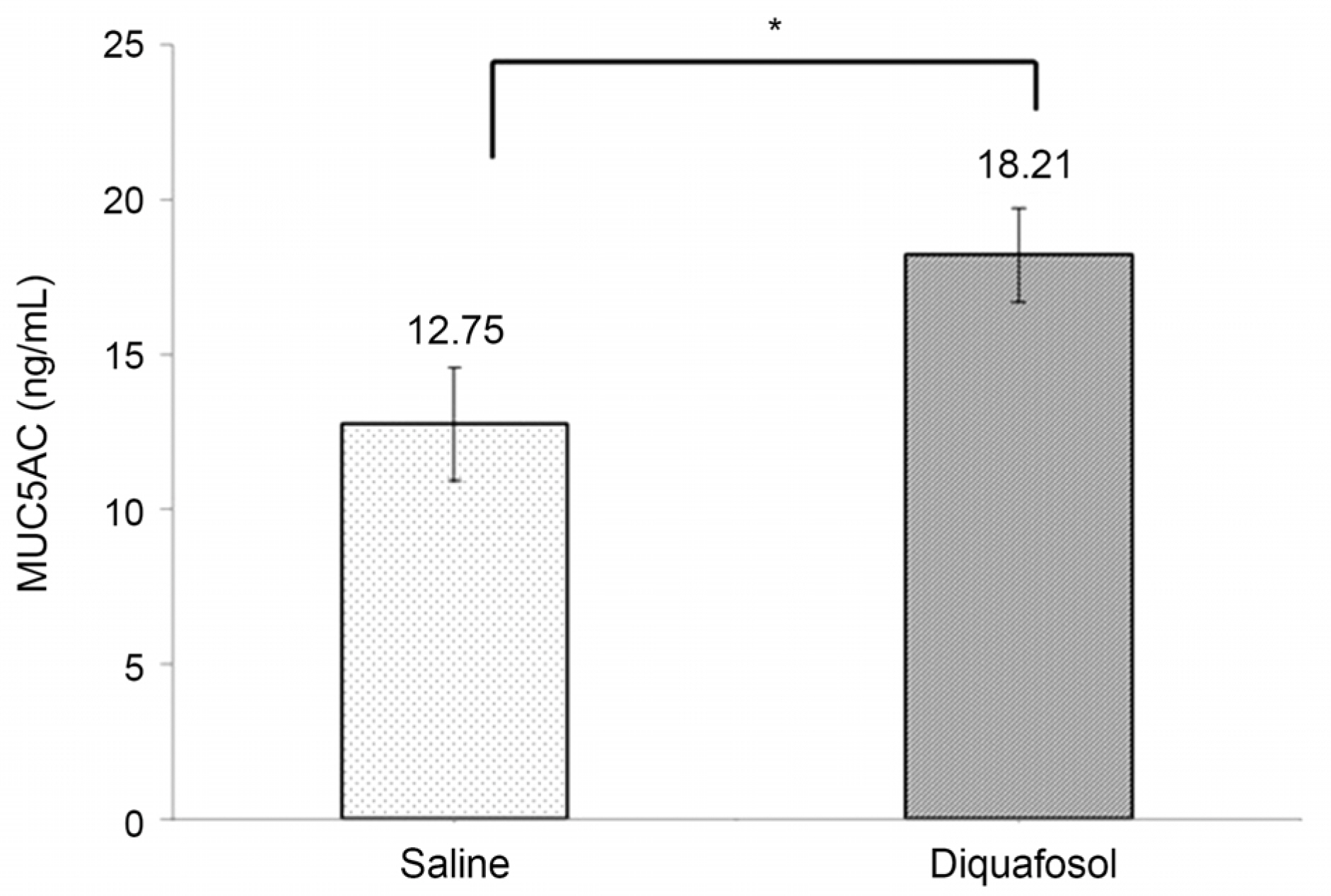
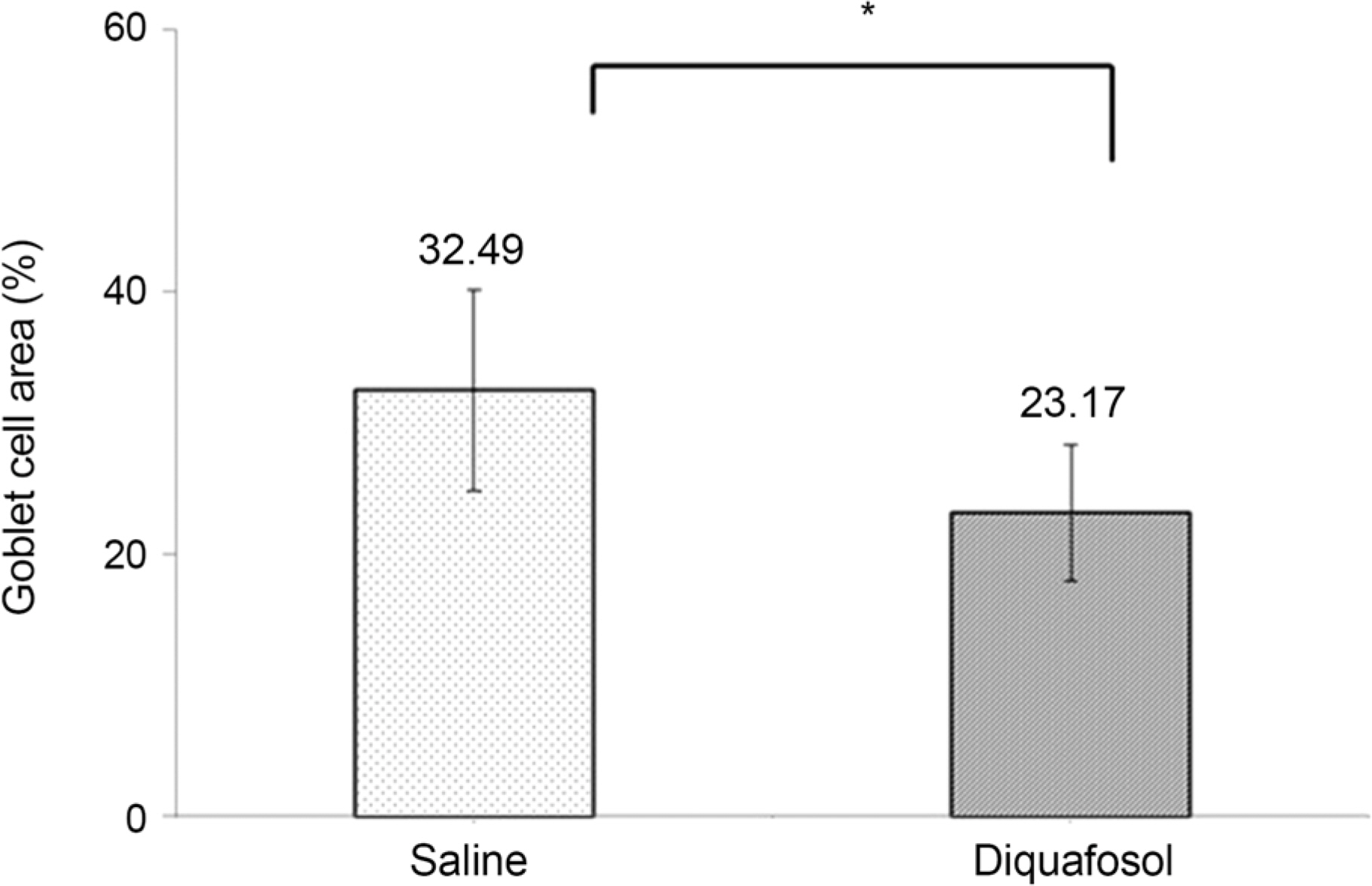
The mean time of corneal wetting property was 86.40 (+/- 17.90) seconds in the diquafosol group and 49.00 (+/- 6.35) seconds in the control group. There was a significant difference between the two groups (p = 0.043). The mean concentration of tear MUC5AC was significantly higher in the diquafosol group (18.21 +/- 1.52 ng/mL) than the control group (12.75 +/- 1.82 ng/mL; p = 0.028). Conjunctival impression cytology showed the area of PAS-stained conjunctival goblet cells was significantly lower in the diquafosol group (23.17 +/- 0.05%) than the control group (32.49 +/- 0.08%; p = 0.028).
CONCLUSIONS
Immediately after instilling 3% diquafosol, corneal wetting property improved significantly. Also tear MUC5AC concentration, which was released from conjunctival goblet cells increased compared to saline.
Keyword
Figure
Cited by 1 articles
-
Effect of 3% Diquafosol Tetrasodium on Tear Film Stability after Laser-assisted in situ Keratomileusis
Chang Hwan Kim, San Seong, Jong Ku Kim, Jae Ho Choi, Chul Myong Choe, Tae Hoon Choi, Sekyung Kim
J Korean Ophthalmol Soc. 2019;60(10):915-921. doi: 10.3341/jkos.2019.60.10.915.
Reference
-
1). Inatomi T, Spurr-Michaud S, Tisdale AS, et al. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996; 37:1684–92.2). Fahmy AM, Hardten DR. Treating ocular surface disease: new agents in development. Clin Ophthalmol. 2011; 5:465–72.3). Jung HH, Kang YS, Sung MS, Yoon KC. Clinical efficacy of topical 3% diquafosol tetrasodium in short tear film break-up time dry eye. J Korean Ophthalmol Soc. 2015; 56:339–44.
Article4). Fujihara T, Murakami T, Nagano T, et al. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002; 18:363–70.
Article5). Fujihara T, Murakami T, Fujita H, et al. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42:96–100.6). Miyake G, Ota I, Miyake K, et al. Effects of topical diquafosol pretreatment on intraoperative corneal wetting. J Cataract Refract Surg. 2014; 40:1682–8.
Article7). Marko CK, Menon BB, Chen G, et al. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013; 183:35–48.
Article8). Woo JM, Jeung WJ, Park WC. Conjunctival epithelial cells auto-cultivated in vivo on human amniotic membrane in rabbits. J Korean Ophthalmol Soc. 2006; 47:812–7.9). Helleboid L, Khatami M, Wei ZG, Rockey JH. Histamine and prostacyclin. Primary and secondary release in allergic conjunctivitis. Invest Ophthalmol Vis Sci. 1991; 32:2281–9.10). Rothman JS, Raizman MB, Friedlaender MH. Seasonal and perennial allergic conjunctivitis. Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. 3rd ed.St Louis: Mosby Elsevier Inc.;2010. p. 567–8.
Article11). Terakado K, Yogo T, Kohara Y, et al. Conjunctival expression of the P2Y2 receptor and the effects of 3% diquafosol ophthalmic solution in dogs. Vet J. 2014; 202:48–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of 3% Diquafosol Tetrasodium on Tear Film Stability after Laser-assisted in situ Keratomileusis
- Clinical Efficacy of Topical 3% Diquafosol Tetrasodium in Short Tear Film Break-Up Time Dry Eye
- Comparison of cytotoxicities and wound healing effects of diquafosol tetrasodium and hyaluronic acid on human corneal epithelial cells
- Comparison of Therapeutic Effects of 3% Diquafosol Tetrasodium with Aging in Dry Eye
- Clinical Efficacy of Topical Diquafosol Tetrasodium after Laser Epithelial Keratomileusis