J Korean Surg Soc.
2010 Mar;78(3):171-176. 10.4174/jkss.2010.78.3.171.
New Assessment of the N1-N2 Substaging in Stage III Colorectal Cancer
- Affiliations
-
- 1Department of Surgery, Seoul Red Cross Hospital, Seoul, Korea.
- 2Department of Surgery, Hansol Hospital, Seoul, Korea.
- 3Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea. gs3945@gmail.com
- KMID: 2211945
- DOI: http://doi.org/10.4174/jkss.2010.78.3.171
Abstract
- PURPOSE
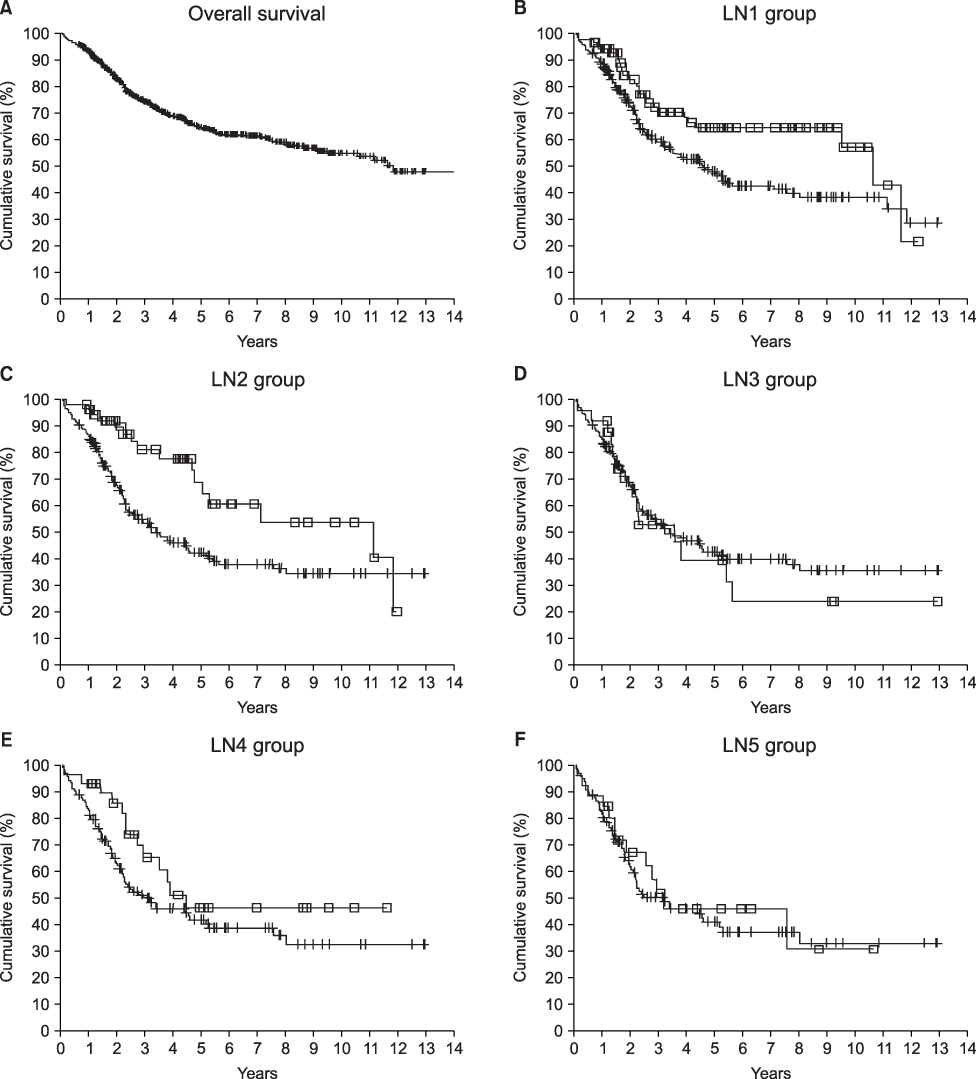
The five-year survival rates of patients with stage III colorectal cancer have been reported widely ranging from 22 to 69 percent. Hence, reliable substaging is important for the management of stage III colorectal cancer patients. Therefore, we tried to assess the substages and investigate the possibility of other discriminating numbers for nodal substaging.
METHODS
The 381 patients with node-positive colorectal cancer who had undergone surgery, were retrospectively categorized by the number of positive nodes. The patients were grouped in five ways, and each grouping was divided into two subgroups according to the number of positive nodes. The subgroups of each grouping were as follows; in LN1 group, N1=1, N2>1; in LN2 group, N1=2, N2>2; in LN3 group, N1=3, N2>3; in LN4 group, N1=4, N2>4; in LN5 group, N1=5, N2>5. We compared the survival rate of each groups.
RESULTS
Node-positive patients had a five-year survival rate of 55.2 percent. The statistical differences between the N1 and N2 subgroups of each grouping were as follows: LN1 group (P=0.0128), LN2 group (P=0.0052), LN3 group (P=0.6268), LN4 group (P=0.1480), and LN5 group (P=0.6875).
CONCLUSION
There were significant differences in the five-year survival rates between N1 and N2 in the LN1 group and LN2 group, but there were no differences between N1 and N2 in the other groupings. These data raise the possibility that a novel N1~N2 substaging (N1: 1~2; N2: >2) is superior to the current N1~N2 substaging (N1: 1~3; N2: >3).
Keyword
Figure
Reference
-
1. Cohen AM, Tremiterra S, Candela F, Thaler HT, Sigurdson ER. Prognosis of node-positive colon cancer. Cancer. 1991. 67:1859–1861.2. Enker WE, Laffer UT, Block GE. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann Surg. 1979. 190:350–360.3. Tang R, Wang JY, Chen JS, Chang-Chien CR, Tang S, Lin SE, et al. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg. 1995. 180:705–712.4. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985. 312:1465–1472.5. Wolmark N, Fisher B, Wieand HS. The prognostic value of the modifications of the Dukes' C class of colorectal cancer. An analysis of the NSABP clinical trials. Ann Surg. 1986. 203:115–122.6. Japanese Research Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Part I. Clinical classification. Jpn J Surg. 1983. 13:557–573.7. Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 2002. 6th ed. New York: Wiley-Liss.8. Kirklin JW, Dockerty MB, Waugh JM. The role of the peritoneal reflection in the prognosis of carcinoma of the rectum and sigmoid colon. Surg Gynecol Obstet. 1949. 88:326–331.9. Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954. 139:846–852.10. Gabriel WB, Dukes C, Bussey HJR. Lymphatic spread in cancer of the rectum. Br J Surg. 1935. 23:395–413.11. Dukes CE, Bussey HJ. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958. 12:309–320.12. Sternberg A, Sibirsky O, Cohen D, Blumenson LE, Rodriguez-Bigas MA, Petrelli NJ. New approach to the substaging of node-positive colorectal adenocarcinoma. Ann Surg Oncol. 1999. 6:161–165.13. Greene FL, Page DL, Fleming ID. AJCC Cancer Staging Manual. 2002. 6th ed. New York: Springer.14. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000. 124:979–994.15. Maurel J, Launoy G, Grosclaude P, Gignoux M, Arveux P, Mathieu-Daude H, et al. Lymph node harvest reporting in patients with carcinoma of the large bowel: a French population-based study. Cancer. 1998. 82:1482–1486.16. Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002. 26:179–189.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Lymph Node Ratio in Stage III Colorectal Cancer
- Total Number of Lymph Nodes Retrieved in Stage III Rectal Cancer Patient
- A Clinical Analysis of the Characteristics of the Lymph Node Metastasis in Patients with Gastrics Cancer
- Postoperative Radiotherapy for Non-Small Cell Lung Cancer
- Reappraisal of AJCC Staging System in Colorectal Cancer