J Korean Soc Transplant.
2015 Mar;29(1):1-8. 10.4285/jkstn.2015.29.1.1.
Immunosuppression in Pediatric Kidney Transplant Patients
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. jwhamd@snu.ac.kr
- 2Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2202557
- DOI: http://doi.org/10.4285/jkstn.2015.29.1.1
Abstract
- Over the last two decades, newer immunosuppressive agents have been introduced in the field of solid organ transplantation, and provided better graft and patient outcome. A wider range of immunosuppressants available to transplant physicians have resulted in improved therapeutic strategies to offer the combinations of medications with non-overlapping toxicities and more suitable immunosuppression. However, only a few clinical trials of new immunosuppressants have been conducted in pediatric patients. This review will discuss the cutting-edge strategy of immunosuppression in children and the current status of new immunosuppressive agents in pre- and post-transplant management to prevent kidney allograft rejection.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Post-Transplant Lymphoproliferative Diseases in Pediatric Kidney Allograft Recipients with Epstein-Barr Virus Viremia
Hyesun Hyun, Eujin Park, Myunghyun Cho, Sang-Il Min, Jongwon Ha, Hyoung Jin Kang, Hee Young Shin, Il-Soo Ha, Hae Il Cheong, Yo Han Ahn, Hee Gyung Kang
J Korean Med Sci. 2019;34(30):. doi: 10.3346/jkms.2019.34.e203.
Reference
-
References
1). Murray JE. The first successful organ transplants in man. J Am Coll Surg. 2005; 200:5–9.
Article2). Horslen S, Barr ML, Christensen LL, Ettenger R, Magee JC. Pediatric transplantation in the United States, 1996–2005. Am J Transplant. 2007; 7(5 Pt 2):1339–58.
Article3). Coelho T, Tredger M, Dhawan A. Current status of immunosuppressive agents for solid organ transplantation in children. Pediatr Transplant. 2012; 16:106–22.
Article4). Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med. 2014; 371:549–58.
Article5). Korean Network for Organ Sharing (KONOS). 2013 Annual Data Report [Internet]. Seoul: KONOS;2014. [cited 2015 Feb 24]. Available from:. http://konos.go.kr.6). Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. OPTN/SRTR 2013 Annual Data Report: Kidney. Am J Transplant. 2015; 15(Suppl 2):1–34.
Article7). North American Pediatric Renal Trials and Collaborative Studies (NAPROTCS). 2010 Annual Transplant Report [Internet]. [cited 2015 Feb 24]. Available from:. https://web.emmes.com/study/ped/annlrept/2010_Report.pdf.8). Kang HG, Kim MS, Park JB, Cho MH, Kim SJ, Ha J, et al. Pediatric kidney transplantation in Korea; experiences over the last thirty years. Seoul: Medrang.9). Church AC. Clinical advances in therapies targeting the interleukin-2 receptor. QJM. 2003; 96:91–102.
Article10). Nagai T, Gotoh Y, Watarai Y, Tajima T, Arai K, Uchida K. Pharmacokinetics and pharmacodynamics of basiliximab in Japanese pediatric renal transplant patients. Int J Clin Pharmacol Ther. 2010; 48:214–23.
Article11). Kovarik JM, Gridelli BG, Martin S, Rodeck B, Melter M, Dunn SP, et al. Basiliximab in pediatric liver transplantation: a pharmacokinetic-derived dosing algorithm. Pediatr Transplant. 2002; 6:224–30.
Article12). Pape L, Strehlau J, Henne T, Latta K, Nashan B, Ehrich JH, et al. Single centre experience with basiliximab in paediatric renal transplantation. Nephrol Dial Transplant. 2002; 17:276–80.
Article13). Duzova A, Buyan N, Bakkaloglu M, Dalgic A, Soylemezoglu O, Besbas N, et al. Triple immunosuppression with or without basiliximab in pediatric renal transplantation: acute rejection rates at one year. Transplant Proc. 2003; 35:2878–80.
Article14). Swiatecka-Urban A, Garcia C, Feuerstein D, Suzuki S, Devarajan P, Schechner R, et al. Basiliximab induction improves the outcome of renal transplants in children and adolescents. Pediatr Nephrol. 2001; 16:693–6.
Article15). National Institute for Clinical Excellence (NICE). Technology appraisal guidance [TA99]: Immunosuppressive therapy for renal transplantation in children and adolescents [Internet]. London: NICE;2006. [cited 2015 Feb 24]. Available from:. https://www.nice.org.uk/guidance/ta99.16). Bartosh SM, Knechtle SJ, Sollinger HW. Campath-1H use in pediatric renal transplantation. Am J Transplant. 2005; 5:1569–73.
Article17). De Serres SA, Mfarrej BG, Magee CN, Benitez F, Ashoor I, Sayegh MH, et al. Immune profile of pediatric renal transplant recipients following alemtuzumab induction. J Am Soc Nephrol. 2012; 23:174–82.
Article18). Shapiro R, Ellis D, Tan HP, Moritz ML, Basu A, Vats AN, et al. Alemtuzumab preconditioning with tacrolimus monotherapy in pediatric renal transplantation. Am J Transplant. 2007; 7:2736–8.
Article19). Tan HP, Donaldson J, Ellis D, Moritz ML, Basu A, Morgan C, et al. Pediatric living donor kidney transplantation under alemtuzumab pretreatment and tacrolimus monotherapy: 4-year experience. Transplantation. 2008; 86:1725–31.
Article20). Supe-Markovina K, Melquist JJ, Connolly D, DiCarlo HN, Waltzer WC, Fine RN, et al. Alemtuzumab with corticosteroid minimization for pediatric deceased donor renal transplantation: a seven-yr experience. Pediatr Transplant. 2014; 18:363–8.
Article21). Li L, Chaudhuri A, Chen A, Zhao X, Bezchinsky M, Concepcion W, et al. Efficacy and safety of thymoglobulin induction as an alternative approach for steroid-free maintenance immunosuppression in pediatric renal transplantation. Transplantation. 2010; 90:1516–20.
Article22). Chavers BM, Chang YC, Gillingham KJ, Matas A. Pediatric kidney transplantation using a novel protocol of rapid (6-day) discontinuation of prednisone: 2-year results. Transplantation. 2009; 88:237–41.
Article23). Moicean AD, Popp AM, Sinescu I. Thymoglobulin–new approaches to optimal outcomes. J Med Life. 2009; 2:319–24.24). Gummert JF, Ikonen T, Morris RE. Newer immunosuppressive drugs: a review. J Am Soc Nephrol. 1999; 10:1366–80.25). Min SI, Ha J, Kim YS, Ahn SH, Park T, Park DD, et al. Therapeutic equivalence and pharmacokinetics of generic tacrolimus formulation in de novo kidney transplant patients. Nephrol Dial Transplant. 2013; 28:3110–9.
Article26). Trompeter R, Filler G, Webb NJ, Watson AR, Milford DV, Tyden G, et al. Randomized trial of tacrolimus versus cyclosporin microemulsion in renal transplantation. Pediatr Nephrol. 2002; 17:141–9.
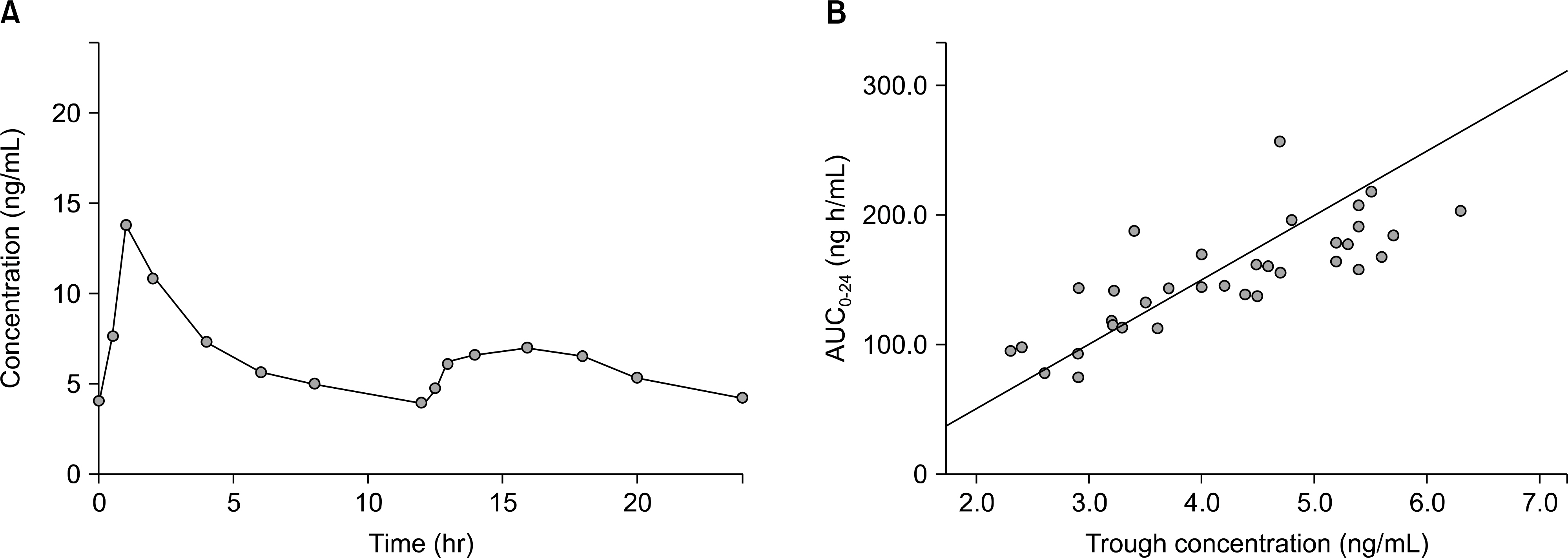
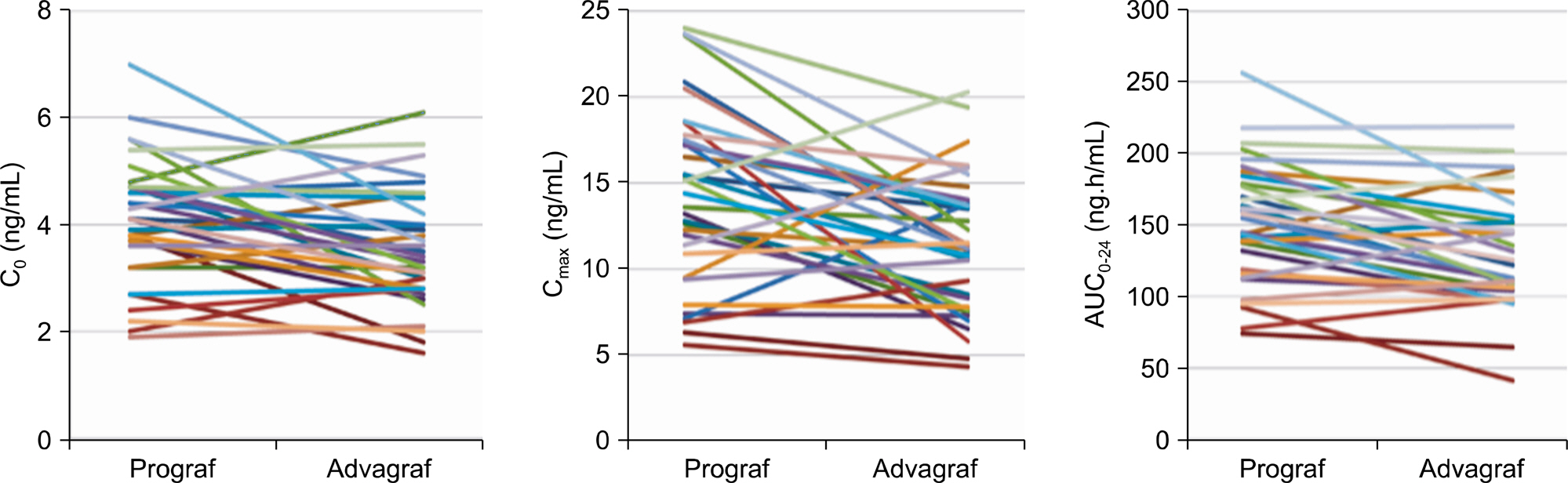
Article27). Krä mer BK, Charpentier B, Bä ckman L, Silva HT Jr, Mondragon-Ramirez G, Cassuto-Viguier E, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant. 2010; 10:2632–43.28). Truneč ka P, Boillot O, Seehofer D, Pinna AD, Fischer L, Ericzon BG, et al. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant. 2010; 10:2313–23.29). Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc. 2005; 37:867–70.
Article30). Wlodarczyk Z, Squifflet JP, Ostrowski M, Rigotti P, Stefoni S, Citterio F, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant. 2009; 9:2505–13.31). Min SI, Ha J, Kang HG, Ahn S, Park T, Park DD, et al. Conversion of twice-daily tacrolimus to once-daily tacrolimus formulation in stable pediatric kidney transplant recipients: pharmacokinetics and efficacy. Am J Transplant. 2013; 13:2191–7.
Article32). Cao W, Mohacsi P, Shorthouse R, Pratt R, Morris RE. Effects of rapamycin on growth factor-stimulated vascular smooth muscle cell DNA synthesis. Inhibition of basic fibroblast growth factor and platelet-derived growth factor action and antagonism of rapamycin by FK506. Transplantation. 1995; 59:390–5.
Article33). Ganschow R, Pape L, Sturm E, Bauer J, Melter M, Gerner P, et al. Growing experience with mTOR inhibitors in pediatric solid organ transplantation. Pediatr Transplant. 2013; 17:694–706.
Article34). Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized longterm trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation. 2004; 77:244–51.
Article35). Basso MS, Subramaniam P, Tredger M, Verma A, Heaton N, Rela M, et al. Sirolimus as renal and immunological rescue agent in pediatric liver transplant recipients. Pediatr Transplant. 2011; 15:722–7.
Article36). Casas-Melley AT, Falkenstein KP, Flynn LM, Ziegler VL, Dunn SP. Improvement in renal function and rejection control in pediatric liver transplant recipients with the introduction of sirolimus. Pediatr Transplant. 2004; 8:362–6.
Article37). Jimé nez-Rivera C, Avitzur Y, Fecteau AH, Jones N, Grant D, Ng VL. Sirolimus for pediatric liver transplant recipients with post-transplant lymphoproliferative disease and hepatoblastoma. Pediatr Transplant. 2004; 8:243–8.38). Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004; 77:1555–61.39). Fricain JC, Cellerie K, Sibaud V, Catros S, Taieb A, Merville P. Oral ulcers in kidney allograft recipients treated with sirolimus. Ann Dermatol Venereol. 2008; 135:737–41.40). Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant. 2008; 8:2607–17.
Article41). Billing H, Rieger S, Sü sal C, Waldherr R, Opelz G, Wü hl E, et al. IVIG and rituximab for treatment of chronic antibody-mediated rejection: a prospective study in paediatric renal transplantation with a 2-year follow-up. Transpl Int. 2012; 25:1165–73.
Article42). Twombley K, Thach L, Ribeiro A, Joseph C, Seikaly M. Acute antibody-mediated rejection in pediatric kidney transplants: a single center experience. Pediatr Transplant. 2013; 17:E149–55.
Article43). Nguyen S, Gallay B, Butani L. Efficacy of bortezomib for reducing donor-specific antibodies in children and adolescents on a steroid minimization regimen. Pediatr Transplant. 2014; 18:463–8.
Article44). Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005; 353:770–81.
Article45). Pestana JO, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S, et al. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012; 12:630–9.46). Reynolds BC, Talbot D, Baines L, Brown A. Use of belatacept to maintain adequate early immunosuppression in calcineurin-mediated microangiopathic hemolysis postrenal transplant. Pediatr Transplant. 2014; 18:E140–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pediatric Liver Transplantation
- A case of disseminated superficial porokeratosis in immunosuppressed kidney transplant recipient
- Squamous Cell Carcinomain Renal Transplant Recipient
- Kidney Retransplantation
- A Case of Post-Transplant Lymphoproliferative Disorder Presenting with Hydronephrosis after Kidney Transplantation