J Korean Radiol Soc.
1998 Mar;38(3):453-458. 10.3348/jkrs.1998.38.3.453.
Squalene Aspiration Pneumonia: Thin-Section CT and Histopathologic Findings
- Affiliations
-
- 1Department of Diagnostic Radiology, Asan Medical Center University of Ulsan College of Medicine.
- 2Department of Diagnostic Pathology, Asan Medical Center University of Ulsan College of Medicine.
- KMID: 2201401
- DOI: http://doi.org/10.3348/jkrs.1998.38.3.453
Abstract
-
PURPOSE: The purpose of this study was to describe the thin-section computed tomography (CT) findings andhistopathologic findings of squalene aspiration pneumonia.
MATERIALS AND METHODS
Thin-section CT scans wereobtained from nine patients with proven exogenous lipoid pneumonia resulting from aspiration of squalene (derivedfrom shark liver oil). The condition was diagnosed by biopsy (n=3), bronchoalveolar lavage (n=4), or sputumcytology and clinical history (n=2); a clinical history of squalene use was confirmed in all patients. Specimensof transbronchial lung biopsy were also reviewed and compared with thin-section CT findings.
RESULTS
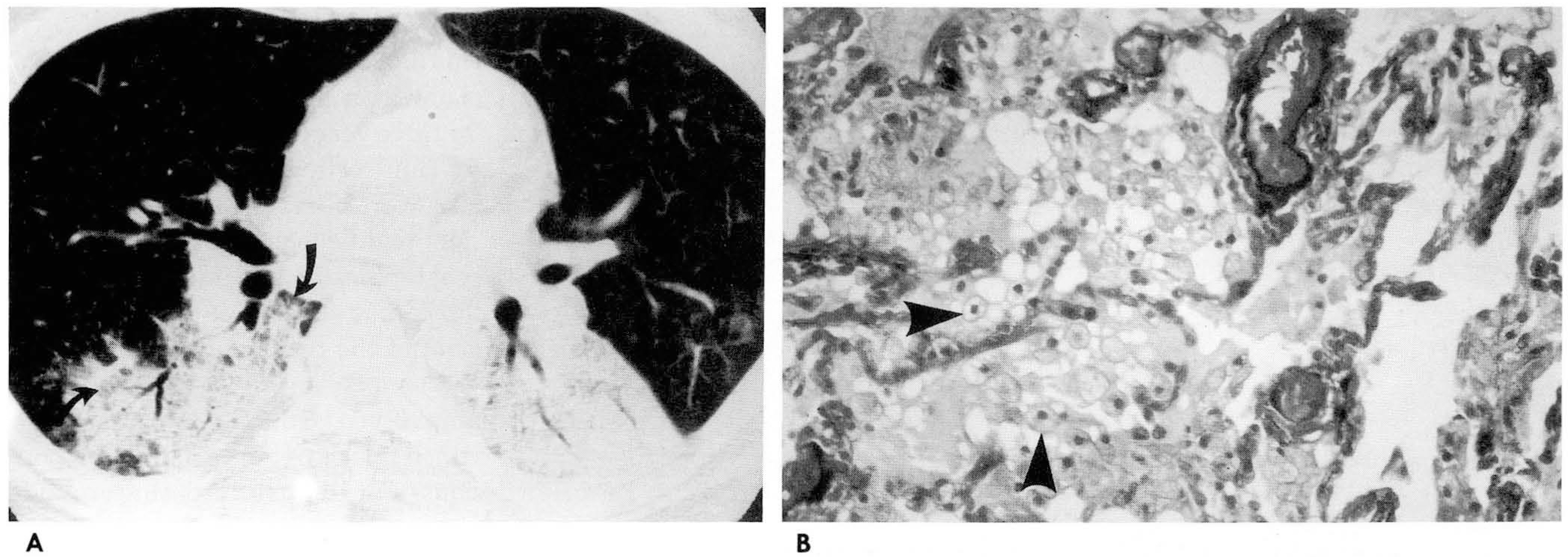
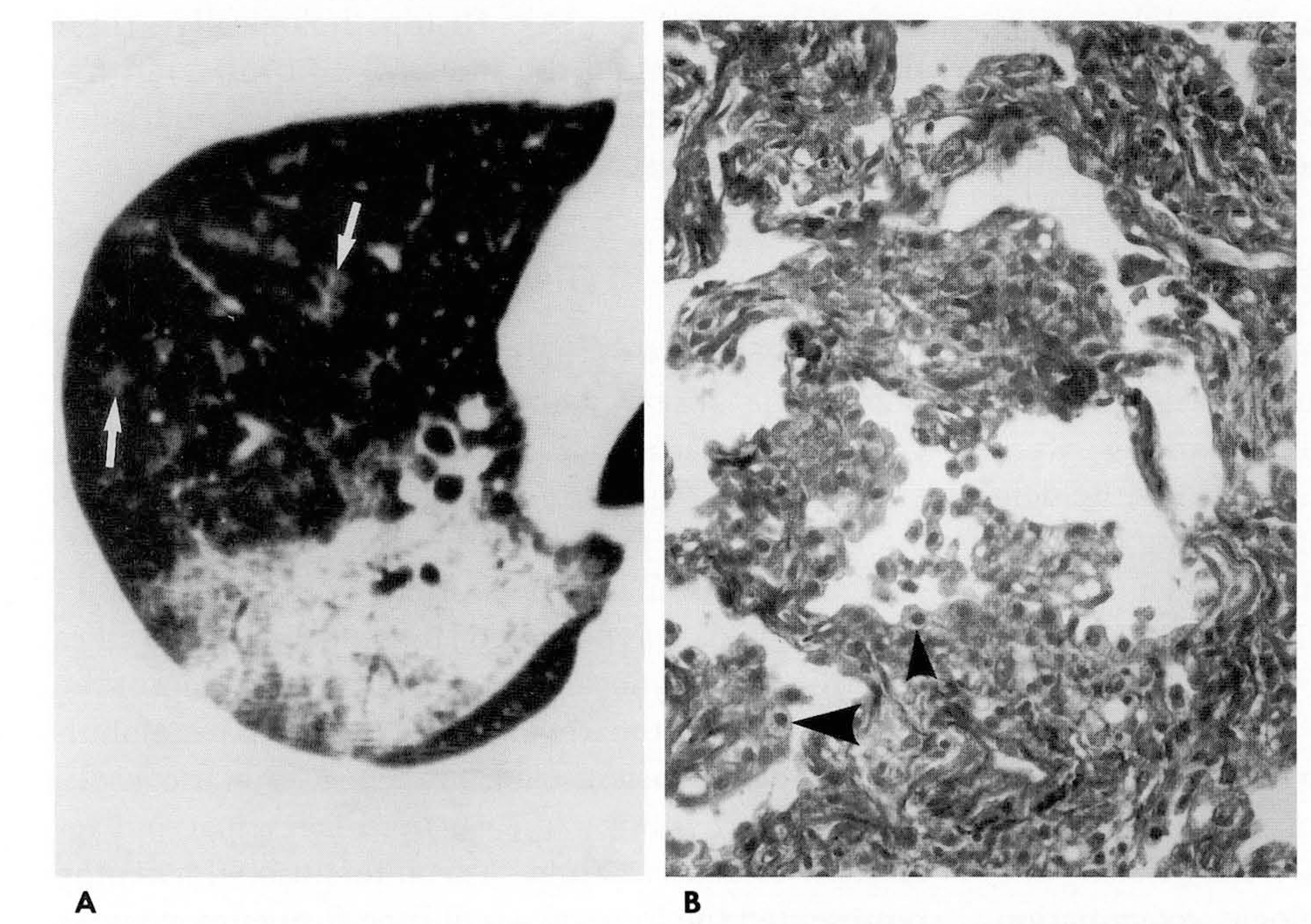
Thin-section CT findings included ground-glass attenuation (n=8), consolidations obliterating vascular marking(n=4), scattered centrilobular ground-glass attenuation (n=2), and interlobular interstitial thickening (n=6).Geographic lobular distribution with peripheral spared lobules was observed in all patients. These diseasesinvolved multiple lobes (n=5) and abnormalities were usually located in the dependent portion of the lung. Inthree cases, histopathological correlation showed that ground-glass attenuation reflected intra-alveolarinfiltration of lipid-laden macrophages with exudative fluid. Interlobular septal thickening representedhyperplasia of type II pneumonocytes with mild fibrosis.
CONCLUSION
On the basis of these results, we concludedthat squalene aspiration pneumonia can be reliably diagnosed by thin-section CT findings particularly when theappropriate history is known.
MeSH Terms
Figure
Reference
-
1.Cannon PR. The problem of lipid pneumonia: a brief review. JAMA. 1940. 115:2176–2179.2.Laughin GF. Studies of pneumonia following naso-pharyngeal injections of oil. Am J Pathol. 1925. 1:407–14.3.Yang JB., Seong HL., Park CS., Park YH., Lee SS. Exogneeous lipoid pneumonia after ingestion of shark liver oil: a case report. J Korean Radiol Soc. 1991. 27:644–646.4.Seo JW., Cho EO., Kim JS., Hur G. MR findings of lipoid pneumonia: report of two cases. J Korean Radiol Soc. 1995. 32:265–268.5.Asnis DS., Saltzman HP., Melchert A. Shark oil pneumonia: An overlooked entity. Chest. 1993. 103:976–977.6.Hugosson CO., Riff EJ., Moore CCM., Akhtar M., Tufenkeji HT. Lipoid pneumonia in infants: a radiographical-pathological study. Pediatr Radiol. 1991. 21:193–197.7.Jenkins DW., Quinn DL. Lipoid pneumonia caused by an Oriental folk medicine. South Med J. 1984. 77:93.
Article8.Lee KS., Muller NL., Hale V., Newell Jr JD., Lynch DA., Im J-G. Lipoid pneumonia: CT findings. J Comput Assist Tomogr. 1995. 19:48–51.9.Budavari S. The Merck Index,. 11th ed.Rahway, Ν J: MERCK & Co. Inc.;1989. p. 1383.10.Brechot JM., Buy JN., Laaban JP., Rochemaure J. Computed tomography and magnetic resonance findings in lipoid pneumonia. Thorax. 1991. 46:738–739.
Article11.Pujol J-L., Barneon G., Bousquet J., Michel F-B., Godard P. Interstitial pulmonary disease induced by occupational exposure to paraffin. Chest. 1990. 97:324–326.
Article12.Wheeler PS., Stitik FP., Jutchins GM., Klinefelter HF., Siegelman SS. Diagnosis of lipoid pneumonia by computed tomography. JAMA. 1981. 245:65–66.
Article13.Godwin JD., Muller NL., Takasugi JE. Pulmonary alveolar proteinosis: CT findings. Radiology. 1988. 169:609–613.
Article14.Jang ΗJ., Lee KS., Kwon Ο J., Rhee CH., Shim YM. Han J (1996) Bronchioloalveolar carcinoma: Focal area of ground-glass attenuation at thin-section CT as an early sign. Radiology. 199:485.15.Kaplan HS (1971) Contiguity and progression in Hodgkin's disease. Cancer Res. 31:1811.16.Lipinski JK., Weisbrod GL., Sanders DE. Exogenous lipoid pneumonitis. Can Assoc Radiol J. 1980. 31:92–98.17.Pinkerton H. The reaction to oils and fats in the lung. Arch Pat hoi. 1928. 5:380–401.18.Brody JS., Levin B. Interlobular septa thickening in lipid pneumonia. AJR. 1962. 88:1061–1069.19.Kennedy JD., Castello P., Balikian JP, et al. Exogenous lipoid pneumonia. AJR. 1940. 1361145–1149.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipoid Pneumonia After Aspiration of Squalene(R) in Rabbit
- Clinical study on lipoid pneumonia caused by aspiration of squalene
- A Case of Lipoid Pneumonia with Hypercalcemia Caused by Squalelne Intake by Force
- Squalene-Induced Lipoid Pneumonia in Rabbits: High-Resolution CT and Pathologic findings
- Hematogenous Candida Pneumonia in Major Burn Patients: Plain Chest Radiograph and Thin-section CT Findings