J Korean Med Assoc.
2014 May;57(5):391-397. 10.5124/jkma.2014.57.5.391.
Use of big data for drug safety monitoring and decision making
- Affiliations
-
- 1Korea Institute of Drug Safety and Risk Management, Seoul, Korea. bjpark@snu.ac.kr
- 2Medical Research Collaborating Center, Seoul National University Hospital, Seoul, Korea.
- 3Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2194881
- DOI: http://doi.org/10.5124/jkma.2014.57.5.391
Abstract
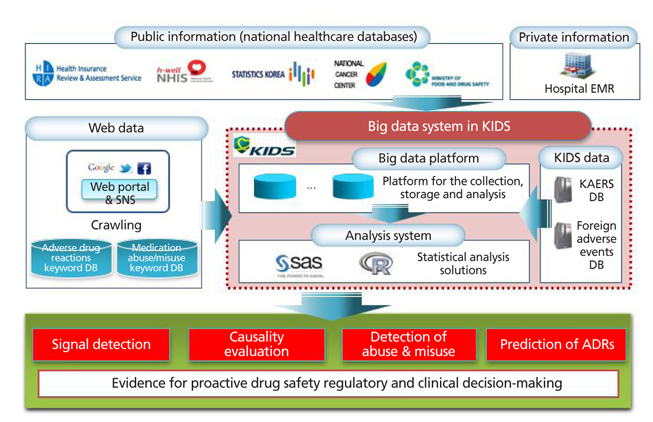
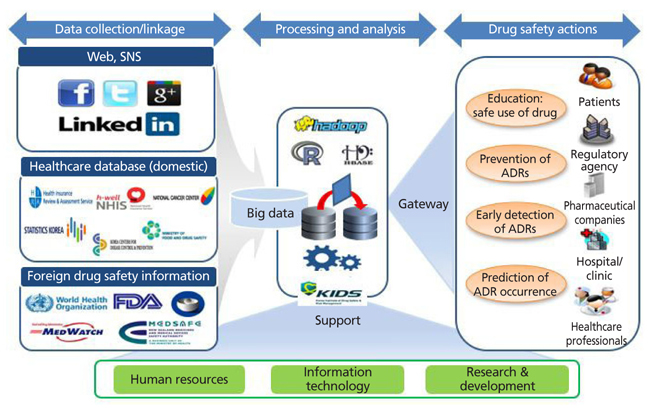
- The development of information technologies has led to the era of big data; such enormous collections of information on drugs and adverse drug reactions are stored in either a structured, a semistructured, or an unstructured form. Because of the nature of the emerging issue of drug safety, it is common for policy makers and healthcare professionals to make decisions without sufficient evidence. Big data may be used as an efficient pharmacovigilance tool, which enables us to recognize adverse drug reactions that may not have been identified in pre-marketing clinical trials, in order to capture the patterns of drug utilization and adverse events, and to predict the occurrence of adverse drug reactions. National surveillance systems using electronic health databases have been established successfully in the US and Europe. The Korea Institute of Drug Safety and Risk Management (KIDS) plans to establish a big data platform for pharmacovigilance in Korea. The big data may be effectively used for evidence-based regulatory and clinical decision making in the field of drug safety and risk management.
Keyword
MeSH Terms
Figure
Reference
-
1. Beyer MA, Douglas L. The importance of 'big data': a definition. Stamford: Gartner;2012.2. Issa NT, Byers SW, Dakshanamurthy S. Big data: the next fron-tier for innovation in therapeutics and healthcare. Expert Rev Clin Pharmacol. 2014; 7:293–298.
Article3. Ginsberg J, Mohebbi MH, Patel RS, Brammer L, Smolinski MS, Brilliant L. Detecting influenza epidemics using search engine query data. Nature. 2009; 457:1012–1014.
Article4. Dugas AF, Jalalpour M, Gel Y, Levin S, Torcaso F, Igusa T, Rothman RE. Influenza forecasting with Google flu trends. PLoS One. 2013; 8:e56176.
Article5. Chung SY, Jung SY, Shin JY, Park BJ. The role of the KIDS for enhancing drug safety and risk management in Korea. J Korean Med Assoc. 2012; 55:861–868.
Article6. Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001; 96:1698–1703.
Article7. Wahab IA, Pratt NL, Kalisch LM, Roughead EE. Comparing time to adverse drug reaction signals in a spontaneous reporting database and a claims database: a case study of rofecoxib-induced myocardial infarction and rosiglitazone-induced heart failure signals in Australia. Drug Saf. 2014; 37:53–64.
Article8. McKeigue PM, Lamm SH, Linn S, Kutcher JS. Bendectin and birth defects: I. A meta-analysis of the epidemiologic studies. Teratology. 1994; 50:27–37.
Article9. Ray WA, Murray KT, Kawai V, Graham DJ, Cooper WO, Hall K, Stein CM. Propoxyphene and the risk of out-of-hospital death. Pharmacoepidemiol Drug Saf. 2013; 22:403–412.
Article10. Hall GC, Sauer B, Bourke A, Brown JS, Reynolds MW, LoCasale R. Guidelines for good database selection and use in pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2012; 21:1–10.
Article11. Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010; 362:865–869.
Article12. Choi NK, Lee J, Park BJ. Recent international initiatives of drug safety management. J Korean Med Assoc. 2012; 55:819–826.
Article13. White RW, Tatonetti NP, Shah NH, Altman RB, Horvitz E. Web-scale pharmacovigilance: listening to signals from the crowd. J Am Med Inform Assoc. 2013; 20:404–408.
Article14. Xu R, Wang Q. Large-scale combining signals from both biomedical literature and the FDA Adverse Event Reporting System (FAERS) to improve post-marketing drug safety signal detection. BMC Bioinformatics. 2014; 15:17.
Article15. Choi NK, Chang Y, Kim JY, Choi YK, Park BJ. Comparison and validation of data-mining indices for signal detection: using the Korean national health insurance claims database. Pharmacoepidemiol Drug Saf. 2011; 20:1278–1286.
Article16. Park MY, Yoon D, Lee K, Kang SY, Park I, Lee SH, Kim W, Kam HJ, Lee YH, Kim JH, Park RW. A novel algorithm for detection of adverse drug reaction signals using a hospital electronic medical record database. Pharmacoepidemiol Drug Saf. 2011; 20:598–607.
Article17. Shin JY, Choi NK, Jung SY, Lee J, Kwon JS, Park BJ. Risk of ischemic stroke with the use of risperidone, quetiapine and olanzapine in elderly patients: a population-based, case-crossover study. J Psychopharmacol. 2013; 27:638–644.
Article18. Choi NK, Lee J, Chang Y, Jung SY, Kim YJ, Lee SM, Lee JH, Kim JY, Song HJ, Park BJ. Polyethylene glycol bowel preparation does not eliminate the risk of acute renal failure: a population-based case-crossover study. Endoscopy. 2013; 45:208–213.
Article19. Song HJ, Lee J, Kim YJ, Jung SY, Kim HJ, Choi NK, Park BJ. β1 selectivity of β-blockers and reduced risk of fractures in elderly hypertension patients. Bone. 2012; 51:1008–1015.
Article20. Jung SY, Choi NK, Kim JY, Chang Y, Song HJ, Lee J, Park BJ. Short-acting nifedipine and risk of stroke in elderly hypertensive patients. Neurology. 2011; 77:1229–1234.
Article21. Choi NK, Hahn S, Park BJ. Increase in mortality rate following coprescription of cisapride and contraindicated drugs. Ann Pharmacother. 2007; 41:667–673.
Article22. Cho S, Sohn CH, Jo MW, Shin SY, Lee JH, Ryoo SM, Kim WY, Seo DW. Correlation between national influenza surveillance data and google trends in South Korea. PLoS One. 2013; 8:e81422.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Shared Decision Making in Geriatric Care
- Evidence-based healthcare and the need of conditional decision
- Predictive Analytics in Spine Oncology Research: First Steps, Limitations, and Future Directions
- Public Health Nurses' Decision Making Models and Their Knowledge Structure
- Case Analysis in Drug Approval by FDA/EMA Using Real-World Data/Real-World Evidence