J Korean Assoc Oral Maxillofac Surg.
2016 Feb;42(1):20-30. 10.5125/jkaoms.2016.42.1.20.
Investigation of a pre-clinical mandibular bone notch defect model in miniature pigs: clinical computed tomography, micro-computed tomography, and histological evaluation
- Affiliations
-
- 1Department of Craniomaxillofacial Regenerative Medicine, The United States Army Dental and Trauma Research Detachment, Fort Sam Houston, USA. teja.guda@utsa.edu
- 2Department of Biomedical Engineering, The University of Texas at San Antonio, San Antonio, TX, USA.
- KMID: 2189406
- DOI: http://doi.org/10.5125/jkaoms.2016.42.1.20
Abstract
OBJECTIVES
To validate a critical-size mandibular bone defect model in miniature pigs.
MATERIALS AND METHODS
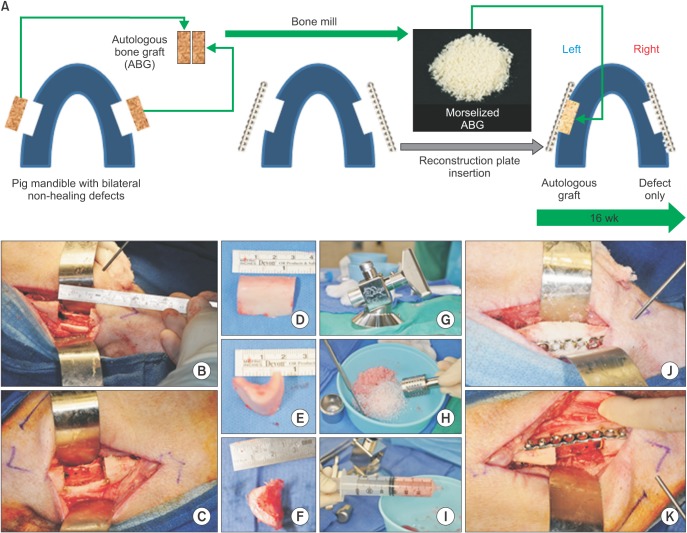
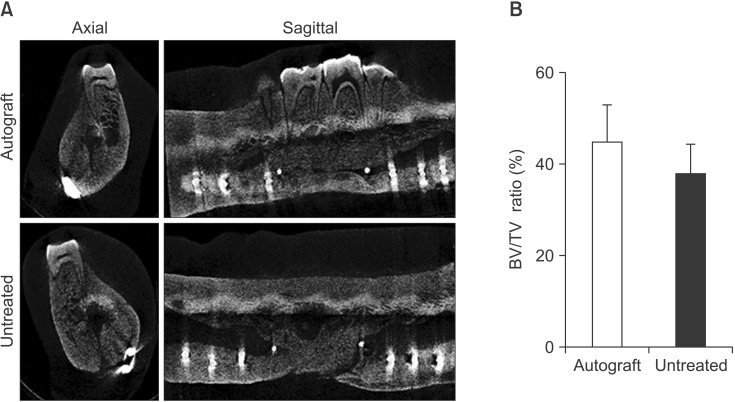
Bilateral notch defects were produced in the mandible of dentally mature miniature pigs. The right mandibular defect remained untreated while the left defect received an autograft. Bone healing was evaluated by computed tomography (CT) at 4 and 16 weeks, and by micro-CT and non-decalcified histology at 16 weeks.
RESULTS
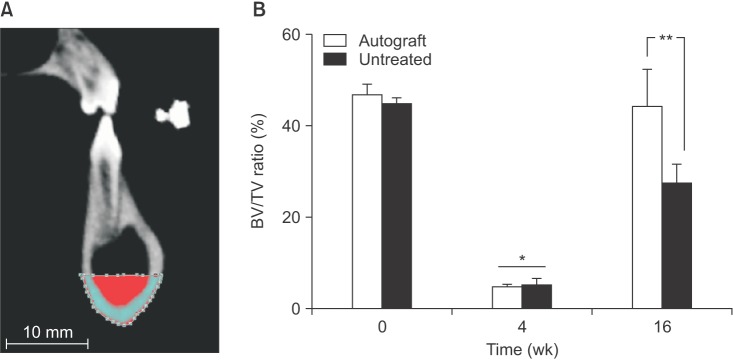
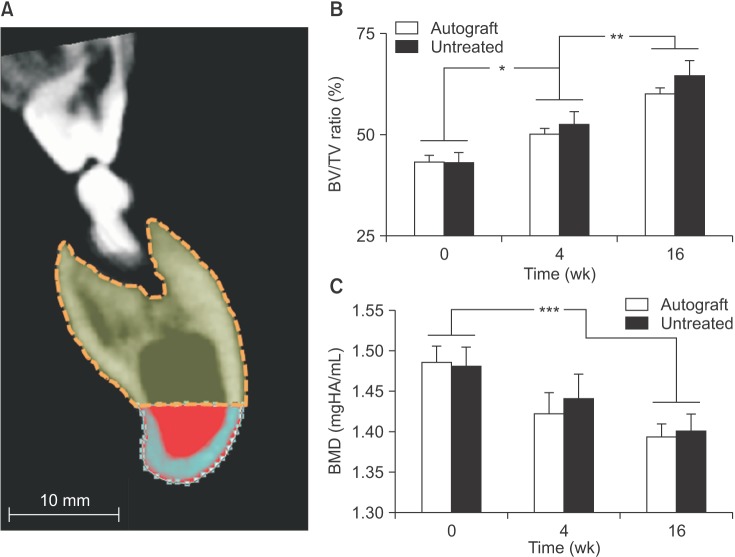
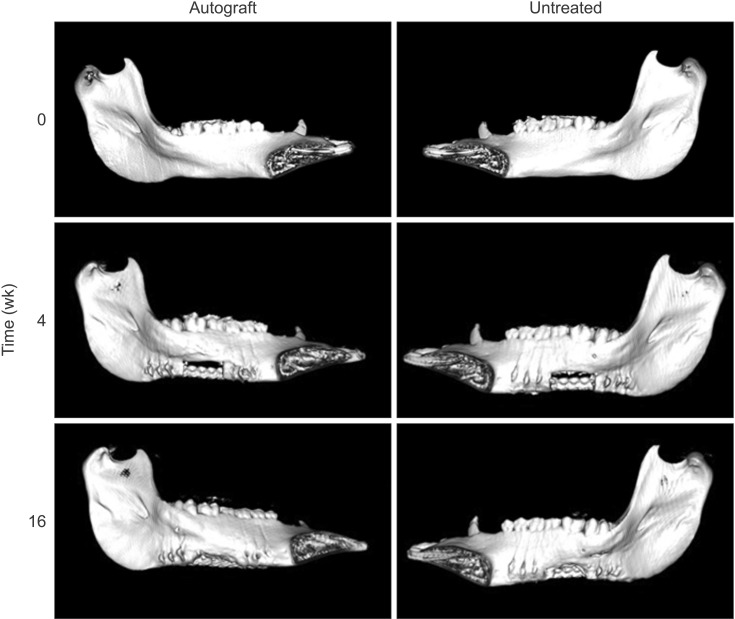
In both the untreated and autograft treated groups, mineralized tissue volume was reduced significantly at 4 weeks post-surgery, but was comparable to the pre-surgery levels after 16 weeks. After 16 weeks, CT analysis indicated that significantly greater bone was regenerated in the autograft treated defect than in the untreated defect (P=0.013). Regardless of the treatment, the cortical bone was superior to the defect remodeled over 16 weeks to compensate for the notch defect.
CONCLUSION
The presence of considerable bone healing in both treated and untreated groups suggests that this model is inadequate as a critical-size defect. Despite healing and adaptation, the original bone geometry and quality of the pre-injured mandible was not obtained. On the other hand, this model is justified for evaluating accelerated healing and mitigating the bone remodeling response, which are both important considerations for dental implant restorations.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Are critical size bone notch defects possible in the rabbit mandible?
Patricia L. Carlisle, Teja Guda, David T. Silliman, Robert G. Hale, Pamela R. Brown Baer
J Korean Assoc Oral Maxillofac Surg. 2019;45(2):97-107. doi: 10.5125/jkaoms.2019.45.2.97.
Reference
-
1. Mitchener TA, Canham-Chervak M. Oral-maxillofacial injury surveillance in the Department of Defense, 1996-2005. Am J Prev Med. 2010; 38(1 Suppl):S86–S93. PMID: 20117604.
Article2. Lew TA, Walker JA, Wenke JC, Blackbourne LH, Hale RG. Characterization of craniomaxillofacial battle injuries sustained by United States service members in the current conflicts of Iraq and Afghanistan. J Oral Maxillofac Surg. 2010; 68:3–7. PMID: 20006147.
Article3. Owens BD, Kragh JF Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi freedom and operation enduring freedom. J Trauma. 2008; 64:295–299. PMID: 18301189.
Article4. Rachmiel A, Srouji S, Peled M. Alveolar ridge augmentation by distraction osteogenesis. Int J Oral Maxillofac Surg. 2001; 30:510–517. PMID: 11829233.
Article5. Petrovic V, Zivkovic P, Petrovic D, Stefanovic V. Craniofacial bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:e1–e9. PMID: 22862985.
Article6. Brown Baer PR, Wenke JC, Thomas SJ, Hale CR. Investigation of severe craniomaxillofacial battle injuries sustained by u.s. Service members: a case series. Craniomaxillofac Trauma Reconstr. 2012; 5:243–252. PMID: 24294409.
Article7. Bahat O, Fontanesi RV, Preston J. Reconstruction of the hard and soft tissues for optimal placement of osseointegrated implants. Int J Periodontics Restorative Dent. 1993; 13:255–275. PMID: 8262722.8. Lekholm U, Zarb GA. Patient selection and preparation. In : Brånemark PI, Zarb GA, Albrektsson T, editors. Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence Publishing;1985. p. 199–209.9. Nasser M, Pandis N, Fleming PS, Fedorowicz Z, Ellis E, Ali K. Interventions for the management of mandibular fractures. Cochrane Database Syst Rev. 2013; 7:CD006087. PMID: 23835608.
Article10. Zakhary IE, El-Mekkawi HA, Elsalanty ME. Alveolar ridge augmentation for implant fixation: status review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114(5 Suppl):S179–S189. PMID: 23063396.
Article11. Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, et al. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009; 30:2149–2163. PMID: 19211141.
Article12. Fennis JP, Stoelinga PJ, Jansen JA. Mandibular reconstruction: a clinical and radiographic animal study on the use of autogenous scaffolds and platelet-rich plasma. Int J Oral Maxillofac Surg. 2002; 31:281–286. PMID: 12190135.
Article13. Fennis JP, Stoelinga PJ, Jansen JA. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg. 2004; 33:48–55. PMID: 14690659.
Article14. Fennis JP, Stoelinga PJ, Jansen JA. Reconstruction of the mandible with an autogenous irradiated cortical scaffold, autogenous corticocancellous bone-graft and autogenous platelet-rich-plasma: an animal experiment. Int J Oral Maxillofac Surg. 2005; 34:158–166. PMID: 15695045.
Article15. Salmon R, Duncan W. Determination of the critical size for nonhealing defects in the mandibular bone of sheep. Part 1: a pilot study. J N Z Soc Periodontol. 1997; (81):6–15. PMID: 9522718.16. Marshall J, Duncan W. Determination of the critical size for nonhealing defects in the mandibular bone of sheep. Part 2: healing of 12 mm circular defects after 16 weeks. J N Z Soc Periodontol. 1997; (82):6–10. PMID: 10483434.17. Ayoub A, Challa SR, Abu-Serriah M, McMahon J, Moos K, Creanor S, et al. Use of a composite pedicled muscle flap and rh-BMP-7 for mandibular reconstruction. Int J Oral Maxillofac Surg. 2007; 36:1183–1192. PMID: 17822878.
Article18. Abu-Serriah M, Kontaxis A, Ayoub A, Harrison J, Odell E, Barbenel J. Mechanical evaluation of mandibular defects reconstructed using osteogenic protein-1 (rhOP-1) in a sheep model: a critical analysis. Int J Oral Maxillofac Surg. 2005; 34:287–293. PMID: 15741038.
Article19. Abu-Serriah MM, Odell E, Lock C, Gillar A, Ayoub AF, Fleming RH. Histological assessment of bioengineered new bone in repairing osteoperiosteal mandibular defects in sheep using recombinant human bone morphogenetic protein-7. Br J Oral Maxillofac Surg. 2004; 42:410–418. PMID: 15336766.
Article20. Gerard D, Carlson ER, Gotcher JE, Jacobs M. Effects of plateletrich plasma on the healing of autologous bone grafted mandibular defects in dogs. J Oral Maxillofac Surg. 2006; 64:443–451. PMID: 16487807.
Article21. Gerard D, Carlson ER, Gotcher JE, Jacobs M. Effects of plateletrich plasma at the cellular level on healing of autologous bonegrafted mandibular defects in dogs. J Oral Maxillofac Surg. 2007; 65:721–727. PMID: 17368369.
Article22. Messora MR, Nagata MJ, Pola NM, de Campos N, Fucini SE, Furlaneto FA. Effect of platelet-rich plasma on bone healing of fresh frozen bone allograft in mandibular defects: a histomorphometric study in dogs. Clin Oral Implants Res. 2013; 24:1347–1353. PMID: 22924970.
Article23. Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990; 1:60–68. PMID: 1965154.
Article24. Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007; 13:1–10. PMID: 17334975.
Article25. Wang S, Liu Y, Fang D, Shi S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007; 13:530–537. PMID: 17944668.
Article26. Jeong HR, Hwang JH, Lee JK. Effectiveness of autogenous tooth bone used as a graft material for regeneration of bone in miniature pig. J Korean Assoc Oral Maxillofac Surg. 2011; 37:375–379.
Article27. Henkel KO, Gerber T, Dietrich W, Bienengräber V. Novel calcium phosphate formula for filling bone defects. Initial in vivo long-term results. Mund Kiefer Gesichtschir. 2004; 8:277–281. PMID: 15480868.28. Henkel KO, Gerber T, Lenz S, Gundlach KK, Bienengräber V. Macroscopical, histological, and morphometric studies of porous bone-replacement materials in minipigs 8 months after implantation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:606–613. PMID: 17052636.
Article29. Ruehe B, Niehues S, Heberer S, Nelson K. Miniature pigs as an animal model for implant research: bone regeneration in criticalsize defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:699–706. PMID: 19782620.
Article30. Ma JL, Pan JL, Tan BS, Cui FZ. Determination of critical size defect of minipig mandible. J Tissue Eng Regen Med. 2009; 3:615–622. PMID: 19731258.
Article31. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–675. PMID: 22930834.
Article32. Otsu N. A threshold selection method from gray-level histograms. Automatica. 1975; 11:23–27.
Article33. Strietzel FP, Khongkhunthian P, Khattiya R, Patchanee P, Reichart PA. Healing pattern of bone defects covered by different membrane types--a histologic study in the porcine mandible. J Biomed Mater Res B Appl Biomater. 2006; 78:35–46. PMID: 16362958.
Article34. Jensen SS, Bornstein MM, Dard M, Bosshardt DD, Buser D. Comparative study of biphasic calcium phosphates with different HA/TCP ratios in mandibular bone defects: a long-term histomorphometric study in minipigs. J Biomed Mater Res B Appl Biomater. 2009; 90:171–181. PMID: 19085941.35. Jensen SS, Broggini N, Hjørting-Hansen E, Schenk R, Buser D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate: a histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res. 2006; 17:237–243. PMID: 16672017.
Article36. Abukawa H, Shin M, Williams WB, Vacanti JP, Kaban LB, Troulis MJ. Reconstruction of mandibular defects with autologous tissueengineered bone. J Oral Maxillofac Surg. 2004; 62:601–606. PMID: 15122567.
Article37. Gröger A, Kläring S, Merten HA, Holste J, Kaps C, Sittinger M. Tissue engineering of bone for mandibular augmentation in immunocompetent minipigs: preliminary study. Scand J Plast Reconstr Surg Hand Surg. 2003; 37:129–133. PMID: 12841611.
Article38. Coen Pramono D. Spontaneous bone regeneration after mandible resection in a case of ameloblastoma: a case report. Ann Acad Med Singapore. 2004; 33(4 Suppl):59–62. PMID: 15389310.39. Ihan Hren N, Miljavec M. Spontaneous bone healing of the large bone defects in the mandible. Int J Oral Maxillofac Surg. 2008; 37:1111–1116. PMID: 18760900.
Article40. Ogunlewe MO, Akinwande JA, Ladeinde AL, Adeyemo WL. Spontaneous regeneration of whole mandible after total mandibulectomy in a sickle cell patient. J Oral Maxillofac Surg. 2006; 64:981–984. PMID: 16713818.
Article41. Zambon R, Mardas N, Horvath A, Petrie A, Dard M, Donos N. The effect of loading in regenerated bone in dehiscence defects following a combined approach of bone grafting and GBR. Clin Oral Implants Res. 2012; 23:591–601. PMID: 22092957.
Article42. Orsini G, Scarano A, Piattelli M, Piccirilli M, Caputi S, Piattelli A. Histologic and ultrastructural analysis of regenerated bone in maxillary sinus augmentation using a porcine bone-derived biomaterial. J Periodontol. 2006; 77:1984–1990. PMID: 17209782.
Article43. Miron RJ, Gruber R, Hedbom E, Saulacic N, Zhang Y, Sculean A, et al. Impact of bone harvesting techniques on cell viability and the release of growth factors of autografts. Clin Implant Dent Relat Res. 2013; 15:481–489. PMID: 22375920.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Are critical size bone notch defects possible in the rabbit mandible?
- Quantification of Microstructures in Mice Alveolar Bone using Micro-computed tomography (microCT)
- Micro-computed tomography analysis of changes in the periodontal ligament and alveolar bone proper induced by occlusal hypofunction of rat molars
- Location and shape of the mandibular lingula: Comparison of skeletal class I and class III patients using panoramic radiography and cone-beam computed tomography
- Evaluation of mandibular cortical bone thickness for placement of temporary anchorage devices (TADs)