J Korean Acad Conserv Dent.
2006 May;31(3):169-178. 10.5395/JKACD.2006.31.3.169.
Time-dependent effects of EDTA application on removal of smear layer in the root canal system
- Affiliations
-
- 1Department of Conservative Dentistry, Division of Dentistry, Graduate School, Kyunghee University, Korea. gwchoi@khu.ac.kr
- KMID: 2175816
- DOI: http://doi.org/10.5395/JKACD.2006.31.3.169
Abstract
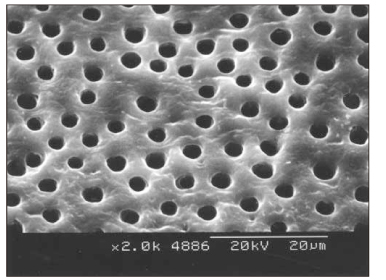
- This study was to verify that the combined application of NaOCl and EDTA was more effective in removal of smear layer than the application of NaOCl alone. Furthermore it was aimed to find out the optimal time for the application of EDTA. Thirty five single rooted teeth were cleaned and shaped. NaOCl solution was used as an irrigant during instrumentation. After instrumentation, root canals of the control group were irrigated with 5 ml of NaOCl for 2 minutes. 30 sec, 1 min, and 2 min group were irrigated with 5 ml of 17% EDTA for 30 sec, 1 min, and 2 min respectively. Then the roots were examined with scanning electron microscopy for evaluating removal of smear layer and erosion of dentinal tubule. The results were as follows; 1. The control group: The smear layer was not removed at all. 2. The other groups: 1) Middle(1/3): All groups showed almost no smear layer. And the erosion occurred more frequently as increasing irrigation time. 2) Apical(1/3): The cleaning effect of 2 min group was better than the others. The results suggest that 2 min application of 17% EDTA should be adequate to remove smear layer on both apical(1/3) and middle(1/3).
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Effect of moisture on sealing ability of root canal filling with different types of sealer through the glucose penetration model
Jin-Ah Jang, Hee-Lyang Kim, Mi-Ja Her, Kwang-Won Lee, Mi-Kyung Yu
J Korean Acad Conserv Dent. 2010;35(5):335-343. doi: 10.5395/JKACD.2010.35.5.335.Apical foramen morphology according to the length of merged canal at the apex
Hee-Ho Kim, Jeong-Bum Min, Ho-Keel Hwang
Restor Dent Endod. 2013;38(1):26-30. doi: 10.5395/rde.2013.38.1.26.
Reference
-
1. Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume flush with several irrigating solutions. Part 3. J Endod. 1983. 9:137–142.
Article2. Gutmann JL, Witherspoon DE. Pathways of the pulp. 1998. 7th ed. St. Louis, Missouri: Mosby Inc.;272.3. Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings or experimentally infected dentinal tubules. Endod Dent Traumatol. 1990. 6:142.
Article4. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982. 15:187–196.
Article5. Ingle JI, Himel VT, Hawrish CE, Glickman GN, Serene T, Rosenberg PA, Buchanan S, West JD, Ruddle CJ, Camp JH, Roane JB, Cecchini SCM. Ingle·Bakland Endodontics. 2002. 5th ed. Hamilton, Ontario: BC Decker Inc.;503–505.6. Russell AD, Hugo WB, Ayliffe GAJ. Principles and Practice of Disinfection, Preservation, and Sterilization. 1999. 3rd ed. Malden, MA: Blackwell Science;99–100.7. Lim TS, Wee TY, Choi MY, Koh WC, Sae-Lim V. Light and scanning electron microscopic evaluation of Glyde™ file Prep in smear layer removal. Int Endod J. 2003. 36:336–343.
Article8. Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985. 18:35–40.
Article9. Hulsmann M, Heckendorff M, Lennon A. Chelating agent in root canal treatment: mode of action and indications for their use. Int Endod J. 2003. 36:810–830.10. Liolios E, Economides N, Parissis-Messimeris S, Boutsioukis A. The effectiveness of three irrigating solutions on root canal cleaning after hand and mechanical preparation. Int Endod J. 1997. 30:51–57.
Article11. Cunningham WT, Balekjian AY. Effect of temperature on collagen-dissolving ability of sodium hypochlorite endodontic irrigant. Oral surg Oral Med Oral Pathol. 1980. 49:175–177.
Article12. Meryon SD, Tobias RS, Jakeman KJ. Smear removal agents: a quantitative study in vivo and in vitro. J Prosthet Dent. 1987. 57:174–179.13. Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003. 29:233–239.
Article14. Tucker JW, Mizrahi S, Seltzer S. Scanning electron microscopic study of the efficacy of various irrigating solutions: Urea, Tublicid Red, and Tubulicid Blue. J Endod. 1976. 2:71–78.
Article15. Love RM, Chandler NP, Jenkinson HF. Penetration of smeared or non smeared dentine by Streptococcus gordonii. Int Endod J. 1996. 29:2–12.
Article16. Ruddle CJ. Pathways of the pulp. 2002. 8th ed. St. Louis, Missouri: Mosby Inc.;258.17. Abou-Rass M, Oglesby SW. The effects of temperature concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod. 1981. 7:376–377.
Article18. Spangberg L, Engstrom B, Langeland K. Biological effects of dental materials. 3 toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral surg Oral Med Oral Pathol. 1973. 36:856–871.19. Patterson SS. In vivo and in vitro studies of the effect of the disodium salt of ethylenediamine tetra-acetate on human dentine and its endodontic implications. Oral surg Oral Med Oral Pathol. 1963. 16:83–103.
Article20. Goldman M, Goldman LB, Cavaleri R, Bogis J, Lin PS. The efficacy of several endodontic irrigating solutions: a scanning electron microscopic study: part 2. J Endod. 1982. 8:487–492.
Article21. White RR, Goldman M, Lin PS. The influence of the smeared layer upon dentinal tubule penetration by plastic filling materials. J Endod. 1984. 10:558–562.
Article22. Grawehr M, Sener B, Waltimo T, Zehnder M. Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int Endod J. 2003. 36:411–415.
Article23. Calt S, Serper A. Smear layer removal by EGTA. J Endod. 2000. 26:459–461.
Article24. Calt S, Serper A. Time-dependant effects of EDTA on dentin structures. J Endod. 2002. 28:17–19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of smear layer treatment on the microleakage
- Removal patterns of smear layer according to application temperature and time of EDTA
- Effects of canal enlargement and irrigation needle depth on the cleaning of the root canal system at 3 mm from the apex
- Smear layer removal by passive ultrasonic irrigation and 2 new mechanical methods for activation of the chelating solution
- Scanning Electron Microscopic Study of the Effect of EDTA on Demineralizing Diseased Root Surface