Investig Magn Reson Imaging.
2015 Sep;19(3):137-145. 10.13104/imri.2015.19.3.137.
Invasive Lobular Carcinoma: MRI Features and Clinicohistological Characteristics According to the ER, PR, and HER2 Statuses
- Affiliations
-
- 1Deparment of Radiology, Gil Hospital, Gachon University School of Medicine and Science, Incheon, Korea. sangyu.nam7@gmail.com
- 2Deparment of Pathology, Gil Hospital, Gachon University School of Medicine and Science, Incheon, Korea.
- KMID: 2175604
- DOI: http://doi.org/10.13104/imri.2015.19.3.137
Abstract
- PURPOSE
To investigate correlations of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2) statuses with magnetic resonance imaging (MRI) features and clinicohistological characteristics in patients with invasive lobular carcinoma (ILC).
MATERIALS AND METHODS
Data from 64 histologically confirmed ILCs were analyzed retrospectively. Preoperative breast MRI was reviewed for morphology and dynamic contrast-enhanced kinetics of the tumor. Pathologic reports were reviewed for ER, PR, and HER2 positivity, tumor size, lymph node metastasis, and the number of metastatic lymph nodes. Furthermore, there was an investigation of the MRI features and clinicohistologic characteristics, according to the ER, PR, and HER2 statuses.
RESULTS
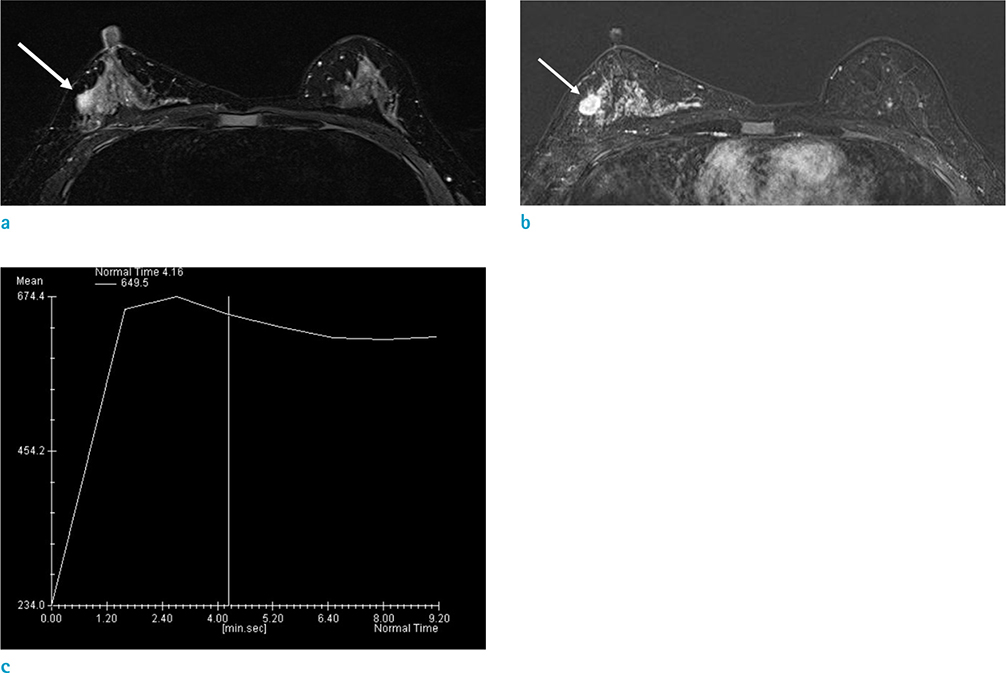
A significant difference in MRI features and clinicohistological tumor characteristics were observed only in relation to PR status. Of the 64 ILCs, 10 (15.6%) were PR negative. PR negative cancers, compared with PR positive cancers, were more likely to present as non-mass enhancement (P = 0.027); have a significantly larger mean tumor size (5.00 +/- 1.05 cm vs. 2.57 +/- 0.21 cm, P = 0.021); and have significantly more metastatic lymph nodes (P = 0.010).
CONCLUSIONS
PR negative ILC presented more frequently as non-mass enhancement on MRI, with larger tumors and increased numbers of metastatic lymph nodes. Therefore, the PR status plays an important role in determining MRI features and clinicohistological characteristics of ILC.
MeSH Terms
Figure
Reference
-
1. Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008; 107:1–14.2. Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003; 289:1421–1424.3. Li CI, Anderson BO, Porter P, Holt SK, Daling JR, Moe RE. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer. 2000; 88:2561–2569.4. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004; 6:R149–R156.5. Rastelli F, Crispino S. Factors predictive of response to hormone therapy in breast cancer. Tumori. 2008; 94:370–383.6. Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008; 26:1419–1426.7. Fernandez-Morales LA, Segui MA, Andreu X, et al. Analysis of the pathologic response to primary chemotherapy in patients with locally advanced breast cancer grouped according to estrogen receptor, progesterone receptor, and HER2 status. Clin Breast Cancer. 2007; 7:559–564.8. Lee SH, Cho N, Kim SJ, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008; 9:10–18.9. Montemurro F, Martincich L, Sarotto I, et al. Relationship between DCE-MRI morphological and functional features and histopathological characteristics of breast cancer. Eur Radiol. 2007; 17:1490–1497.10. Szabo BK, Aspelin P, Kristoffersen Wiberg M, Tot T, Bone B. Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur Radiol. 2003; 13:2425–2435.11. Teifke A, Behr O, Schmidt M, et al. Dynamic MR imaging of breast lesions: correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology. 2006; 239:351–360.12. Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® Magnetic Resonance Imaging. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology;2013.13. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003; 21:1973–1979.14. Anderson H, Hills M, Zabaglo L, et al. Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Ann Oncol. 2011; 22:1770–1776.15. Chen L, Romond E, Chokshi S, et al. A prognostic model of early breast cancer relapse after standard adjuvant therapy and comparison with metastatic disease on initial presentation. Breast Cancer Res Treat. 2012; 136:565–572.16. Nishimura R, Osako T, Nishiyama Y, et al. Evaluation of factors related to late recurrence--later than 10 years after the initial treatment--in primary breast cancer. Oncology. 2013; 85:100–110.17. Gelbfish GA, Davidson AL, Kopel S, et al. Relationship of estrogen and progesterone receptors to prognosis in breast cancer. Ann Surg. 1988; 207:75–79.18. Knopfelmacher A, Fox J, Lo Y, Shapiro N, Fineberg S. Correlation of histopathologic features of ductal carcinoma in situ of the breast with the oncotype DX DCIS score. Mod Pathol. 2015; [Epub ahead of print].19. Stierer M, Rosen H, Weber R, Hanak H, Spona J, Tuchler H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann Surg. 1993; 218:13–21.20. Dhakal HP, Naume B, Synnestvedt M, et al. Vascularization in primary breast carcinomas: its prognostic significance and relationship with tumor cell dissemination. Clin Cancer Res. 2008; 14:2341–2350.21. Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat. 2015; 149:727–741.22. Chen JH, Nalcioglu O, Su MY. MR imaging features of invasive breast cancer correlated with hormonal receptors: does progesterone receptor matter? Ann Oncol. 2008; 19:1024–1026.23. Chen JH, Baek HM, Nalcioglu O, Su MY. Estrogen receptor and breast MR imaging features: a correlation study. J Magn Reson Imaging. 2008; 27:825–833.24. Aitken E, Osman M. Factors affecting nodal status in invasive breast cancer: a retrospective analysis of 623 patients. Breast J. 2010; 16:271–278.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nodular Metastatic Carcinoma from Invasive Lobular Breast Cancer
- Clinical Analysis of an Invasive Lobular Carcinoma in the Breast
- Changes in the Hormone Receptors and the HER2 Expression in Primary and Recurrent Breast Cancer
- Clinicopathologic Characteristics of Apocrine Breast Carcinoma
- Branched-chain Assay for ER, PR, and HER2 RNA Levels is a Useful Adjunct in the Evaluation of ER, PR, and HER2 in Breast Cancer