Diabetes Metab J.
2011 Jun;35(3):290-297. 10.4093/dmj.2011.35.3.290.
Retrospective Analysis on the Efficacy, Safety and Treatment Failure Group of Sitagliptin for Mean 10-Month Duration
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. cydoctor@chol.com
- KMID: 2175440
- DOI: http://doi.org/10.4093/dmj.2011.35.3.290
Abstract
- BACKGROUND
To investigate the clinical results of sitagliptin (SITA) and the characteristics of the treatment failure group or of low responders to SITA.
METHODS
A retrospective study of type 2 diabetic patients reviewed 99 cases, including 12 treatment failure cases, who stopped SITA because of worsening patients' condition, and 87 cases, who continued treatment over five visits (total 9.9+/-10.1 months) after receiving the prescription of SITA from December 2008 to June 2009. Subjects were classified as five groups administered SITA as an initial combination with metformin (MET), add-on to metformin or sulfonylurea, and switching from sulfonylurea or thiazolidinedione. The changes in HbA1c level from the first to last visit (DeltaHbA1c) in treatment maintenance group were subanalyzed.
RESULTS
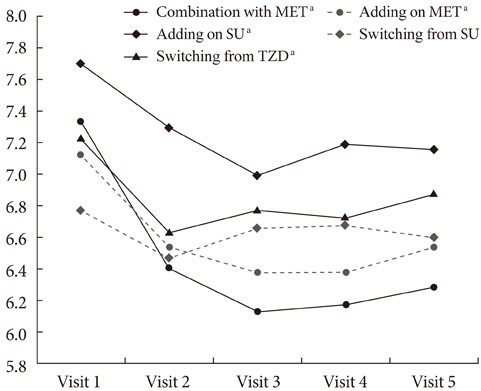
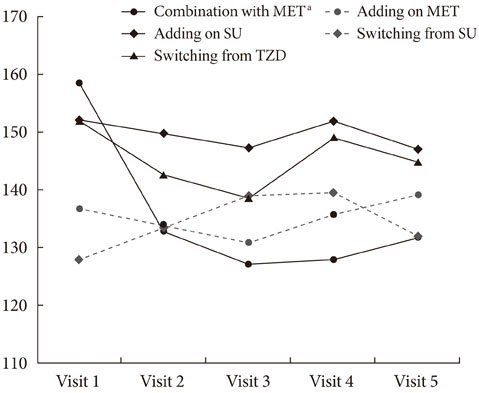
The HbA1c level was significantly reduced in four groups, including initial coadministration of SITA with metformin (DeltaHbA1c=-1.1%, P<0.001), add-on to MET (DeltaHbA1c=-0.6%, P=0.017), add-on to sulfonylurea (DeltaHbA1c=-0.5%, P<0.001), and switching from thiazolidinedione (DeltaHbA1c=-0.3%, P=0.013). SITA was noninferior to sulfonlyurea (DeltaHbA1c=-0.2%, P=0.63). There was no significant adverse effect. The treatment failure group had a longer diabeties duration (P=0.008), higher HbA1c (P=0.001) and fasting plasma glucose (P=0.003) compared to the maintenance group. Subanalysis on the tertiles of DeltaHbA1c showed that low-response to SITA (tertile 1) was associated with a longer diabetes duration (P=0.009) and lower HbA1c (P<0.001).
CONCLUSION
SITA was effective and safe for use in Korean type 2 diabetic patients. However, its clinical responses and long-term benefit-harm profile is yet to be established.
MeSH Terms
Figure
Reference
-
1. Yoon KH. Pathogenesis of type 2 diabetes in Korea. J Korean Diabetes Assoc. 2000. 24:397–403.2. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000. 23:Suppl 2. B21–B29.3. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999. 281:2005–2012.4. Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, Lasseter KC, Kipnes MS, Snyder K, Hilliard D, Tanen M, Cilissen C, De Smet M, de Lepeleire I, Van Dyck K, Wang AQ, Zeng W, Davies MJ, Tanaka W, Holst JJ, Deacon CF, Gottesdiener KM, Wagner JA. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006. 91:4612–4619.5. Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002. 143:4397–4408.6. Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003. 144:5149–5158.7. Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001. 86:3717–3723.8. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993. 91:301–307.9. Kim MH, Lee MK. Incretins and pancreatic beta cells: use of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide to cure type 2 diabetes mellitus. Korean Diabetes J. 2010. 34:2–9.10. Dhillon S. Sitagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2010. 70:489–512.11. Rosenstock J, Zinman B. Dipeptidyl peptidase-4 inhibitors and the management of type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2007. 14:98–107.12. Ahren B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007. 30:1344–1350.13. Mistry GC, Bergman AJ, Zheng W, Hreniuk D, Zinny MA, Gottesdiener KM, Wagner JA, Herman GA, Ruddy M. Sitagliptin, an dipeptidyl peptidase-4 inhibitor, does not alter the pharmacokinetics of the sulphonylurea, glyburide, in healthy subjects. Br J Clin Pharmacol. 2008. 66:36–42.14. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007. 30:1979–1987.15. Williams-Herman D, Johnson J, Teng R, Luo E, Davies MJ, Kaufman KD, Goldstein BJ, Amatruda JM. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin. 2009. 25:569–583.16. Qi DS, Teng R, Jiang M, Davies MJ, Kaufman KD, Amatruda JM, Williams-Herman D. Two-year treatment with sitagliptin and initial combination therapy of sitagliptin and metformin provides substantial and durable glycaemic control in patients with type 2 diabetes. Diabetologia. 2008. 51:Suppl 1. S36.17. Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006. 29:2638–2643.18. Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, Langdon RB, Stein PP, Alba M. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008. 24:537–550.19. Scott R, Loeys T, Davies MJ, Engel SS. Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008. 10:959–969.20. Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007. 9:733–745.21. Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007. 9:194–205.22. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007. 298:194–206.23. Vilsboll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD, Amatruda JM, Engel SS, Katz L. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010. 12:167–177.24. Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004. 18:439–456.25. Brubaker PL, Anini Y. Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can J Physiol Pharmacol. 2003. 81:1005–1012.26. Beinborn M, Worrall CI, McBride EW, Kopin AS. A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regul Pept. 2005. 130:1–6.27. Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006. 29:2632–2637.28. Mohan V, Yang W, Son HY, Xu L, Noble L, Langdon RB, Amatruda JM, Stein PP, Kaufman KD. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009. 83:106–116.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination of synbiotic and sitagliptin in nonalcoholic fatty liver disease: Is it better than sitagliptin alone?
- Efficacy of Sitagliptin When Added to Ongoing Therapy in Korean Subjects with Type 2 Diabetes Mellitus
- Factors Influencing Glycemic Control Response of Sitagliptin
- Adalimumab Treatment in Pediatric-Onset Crohn's Disease Patients after Infliximab Failure: A Single Center Study
- Predictive Clinical Parameters for the Therapeutic Efficacy of Sitagliptin in Korean Type 2 Diabetes Mellitus