Intest Res.
2014 Jan;12(1):53-59. 10.5217/ir.2014.12.1.53.
Comparison of the Efficacy and Tolerability between Same-day Picosulfate and Split-dose Polyethylene Glycol Bowel Preparation for Afternoon Colonoscopy: A Prospective, Randomized, Investigator-blinded Trial
- Affiliations
-
- 1Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea. kto0440@paik.ac.kr
- KMID: 2174338
- DOI: http://doi.org/10.5217/ir.2014.12.1.53
Abstract
- BACKGROUND/AIMS
In the present study, we evaluated the efficacy and tolerability between same-day bowel preparation protocols using 2 sachets of Picosulfate and a 4 L split-dose polyethylene glycol (PEG) bowel preparation for afternoon colonoscopy.
METHODS
The study had a single-center, prospective, randomized, and investigator-blinded, non-inferiority design. We evaluated bowel preparation quality according to the Ottawa scale, patient tolerability, compliance, incidence of adverse events, sleep quality, and polyp/adenoma detection rate.
RESULTS
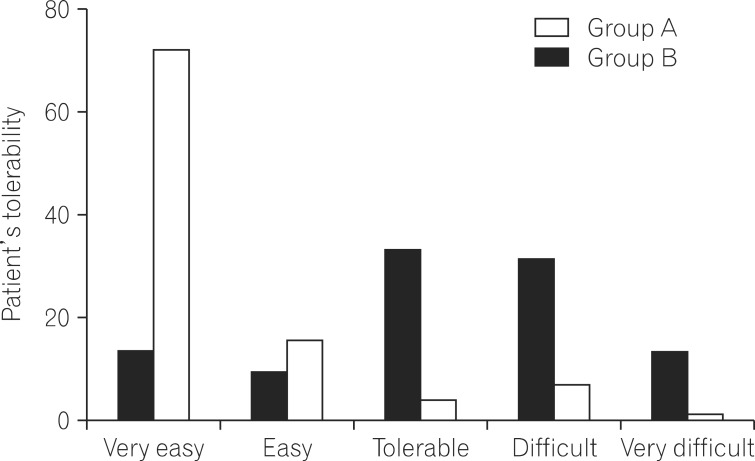
Among the 196 patients analyzed (mean age, 55.3 years; 50.3% men), 97 received the same-day regimen of 2 sachets of picosulfate (group A) and 99 received the 4 L split-dose PEG regimen (group B). The Ottawa score of the total colon was 4.05+/-1.56 in group A and 3.80+/-1.55 in group B (P=0.255). The proportion of patients having adequate bowel preparation in the same-day picosulfate group (61.5%) was slightly less than the 4 L PEG group (71.3%); however, the difference was not statistically significant (P=0.133). Tolerability of the group A regimen was superior to that of the group B regimen (P<0.000). The same-day picosulfate regimen was associated with fewer adverse events, such as abdominal bloating (P=0.037) and better sleep quality (P<0.000).
CONCLUSIONS
The same-day picosulfate regimen and the 4 L split-dose PEG regimen had similar efficacy in bowel preparation for afternoon colonoscopy. However, the same-day picosulfate regimen was easier to administer, produced fewer adverse events, and enabled better sleep quality.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Bowel Preparation, the First Step for a Good Quality Colonoscopy
Ho-Su Lee, Jeong-Sik Byeon
Intest Res. 2014;12(1):1-2. doi: 10.5217/ir.2014.12.1.1.Colon Transit Time May Predict Inadequate Bowel Preparation in Patients With Chronic Constipation
Hong Jun Park, Myeong Hun Chae, Hyun-Soo Kim, Jae Woo Kim, Moon Young Kim, Soon Koo Baik, Sang Ok Kwon, Hee Man Kim, Kyong Joo Lee
Intest Res. 2015;13(4):339-345. doi: 10.5217/ir.2015.13.4.339.
Reference
-
1. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000; 343:162–168. PMID: 10900274.
Article2. Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993; 329:1977–1981. PMID: 8247072.
Article3. Harewood GC, Wiersema MJ, Melton LJ 3rd. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002; 97:3186–3194. PMID: 12492209.
Article4. Chiu HM, Lin JT, Wang HP, Lee YC, Wu MS. The impact of colon preparation timing on colonoscopic detection of colorectal neoplasms--a prospective endoscopist-blinded randomized trial. Am J Gastroenterol. 2006; 101:2719–2725. PMID: 17026559.
Article5. Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005; 61:378–384. PMID: 15758907.
Article6. Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006; 101:2866–2877. PMID: 17227527.
Article7. Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990; 85:422–427. PMID: 2183591.8. Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointest Endosc. 2002; 56:895–902. PMID: 12447305.
Article9. Ahmed M, Raval P, Buganza G. Oral sodium phosphate catharsis and acute renal failure. Am J Gastroenterol. 1996; 91:1261–1262. PMID: 8651185.10. Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs. 2009; 69:123–136. PMID: 19192941.11. Clark LE, Dipalma JA. Safety issues regarding colonic cleansing for diagnostic and surgical procedures. Drug Saf. 2004; 27:1235–1242. PMID: 15588118.
Article12. DiPalma JA, Buckley SE, Warner BA, Culpepper RM. Biochemical effects of oral sodium phosphate. Dig Dis Sci. 1996; 41:749–753. PMID: 8674396.
Article13. Longcroft-Wheaton G, Bhandari P. Same-day bowel cleansing regimen is superior to a split-dose regimen over 2 days for afternoon colonoscopy: results from a large prospective series. J Clin Gastroenterol. 2012; 46:57–61. PMID: 22064553.
Article14. Rex DK, Di Palma JA, Rodriguez R, McGowan J, Cleveland M. A randomized clinical study comparing reduced-volume oral sulfate solution with standard 4-liter sulfate-free electrolyte lavage solution as preparation for colonoscopy. Gastrointest Endosc. 2010; 72:328–336. PMID: 20646695.
Article15. Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004; 59:482–486. PMID: 15044882.
Article16. Seo EH, Kim TO, Park MJ, et al. Optimal preparation-to-colonoscopy interval in split-dose PEG bowel preparation determines satisfactory bowel preparation quality: an observational prospective study. Gastrointest Endosc. 2012; 75:583–590. PMID: 22177570.
Article17. Seo EH, Kim TO, Kim TG, et al. Efficacy and tolerability of split-dose PEG compared with split-dose aqueous sodium phosphate for outpatient colonoscopy: a randomized, controlled trial. Dig Dis Sci. 2011; 56:2963–2971. PMID: 21656179.
Article18. Di Palma JA, Rodriguez R, McGowan J, Cleveland M. A randomized clinical study evaluating the safety and efficacy of a new, reduced-volume, oral sulfate colon-cleansing preparation for colonoscopy. Am J Gastroenterol. 2009; 104:2275–2284. PMID: 19584830.
Article19. Poon CM, Lee DW, Mak SK, et al. Two liters of polyethylene glycol-electrolyte lavage solution versus sodium phosphate as bowel cleansing regimen for colonoscopy: a prospective randomized controlled trial. Endoscopy. 2002; 34:560–563. PMID: 12170410.
Article20. Eun CS, Han DS, Hyun YS, et al. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2011; 56:539–544. PMID: 21042853.
Article21. Hosoe N, Nakashita M, Imaeda H, et al. Comparison of patient acceptance of sodium phosphate versus polyethylene glycol plus sodium picosulfate for colon cleansing in Japanese. J Gastroenterol Hepatol. 2012; 27:1617–1622. PMID: 22646064.
Article22. Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, et al. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006; 12:6161–6166. PMID: 17036388.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal and Safe Bowel Preparation for Colonoscopy
- A Randomized Prospective Trial Comparing a New Polyethylene Glycol Based Lavage Solution with the Standard Polyethylene Glycol Solution in the Preparation of Patients Undergoing Colonoscopy (Clinical trial of new PEG solution in bowel preparation)
- Split-dose Bowel Preparation for Colonoscopy: 2 Liters Polyethylene Glycol with Ascorbic Acid versus Sodium Picosulfate versus Oral Sodium Phosphate Tablets
- Comparision of Single Versus Split-dose of Polyethylene Glycol-electrolyte Solution for Colonoscopy Preparation
- Sulfate free polyethylene glycol versus standard polyethylene glycol for colonoscopy preparation: a prospective, randomized, investigator-blinded comparison