Ann Dermatol.
2008 Jun;20(2):56-66. 10.5021/ad.2008.20.2.56.
The Effect of Gromwell (Lithospermum erythrorhizon) Extract on the Stratum Corneum Hydration and Ceramides Content in Atopic Dermatitis Patients
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Kyung Hee University, Seoul, Korea. nikim@khmc.or.kr
- 2Department of Medical Nutrition, Graduate School of East-West Medical Science, Kyung Hee University, Seoul, Korea.
- 3Amorepacific Corporation R & D Center, Yongin, Korea.
- 4Nutrex Co., Ltd, Seoul, Korea.
- KMID: 2172085
- DOI: http://doi.org/10.5021/ad.2008.20.2.56
Abstract
-
BACKGROUND: A disruption of the balance between the water content of the stratum corneum (SC) and skin surface lipids may lead to the clinical manifestation of dryness of skin in patients with atopic dermatitis (AD).
OBJECTIVE
To determine whether supplementation of gromwell (Lithospermum erythrorhizon), one of herbs used in East Asia in remedies for various abnormal skin conditions, may improve the SC level of hydration and ceramides, major lipid in SC in patients with AD.
METHODS
A total of 28 subjects with AD were randomly assigned into two groups: either gromwell group received dextrose contained capsules with 1.5 g of gromwell extracts or placebo group received only dextrose contained capsules for 10 weeks.
RESULTS
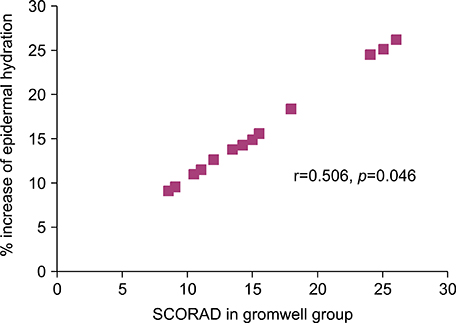
In contrast to no alteration of SC hydration and ceramides in placebo group, the SC hydration in gromwell group was significantly increased in parallel with an increase of SC ceramides. Furthermore, % increase of SC hydration in gromwell group bore a positive correlation with the clinical severity, which suggests that the increase of SC hydration in gromwell group was more effective as AD was more severe.
CONCLUSION
Supplementation of gromwell improves SC hydration in parallel with an increase of ceramides in part.
Keyword
MeSH Terms
Figure
Reference
-
1. Gray GM, White RJ, Williams RH, Yardley HJ. Lipid composition of the superficial stratum corneum cells of the epidermis. Br J Dermatol. 1982; 106:59–63.
Article2. Hedberg CL, Wertz PW, Downing DT. The time course of lipid biosynthesis in pig epidermis. J Invest Dermatol. 1988; 91:169–174.
Article3. Grubauer G, Feingold KR, Harris RM, Elias PM. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989; 30:89–96.
Article4. Wertz PW, Cho ES, Downing DT. Effect of essential fatty acid deficiency on the epidermal sphingolipids of the rats. Biochim Biophys Acta. 1983; 753:350–355.
Article5. Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased levels of ceramides in stratum corneum of atopic dermatitis: an etiological factor in atopic dry skin. J Invest Dermatol. 1991; 96:523–526.
Article6. Matsumoto M, Umemoto N, Sugiura H, Uehara M. Difference in ceramide composition between "dry" and normal skin in patients with atopic dermatitis. Acta Derm Venereol. 1999; 79:246–247.
Article7. Aioi A, Tonogaito H, Suto H, Hamada K, Ra CR, Ogawa H, et al. Impairment of skin barrier function in NC/Nga Tnd mice as a possible model for atopic dermatitis. Br J Dermatol. 2001; 144:12–18.
Article8. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005; 125:183–200.
Article9. Tanaka S, Tajima M, Tsujada M, Tabata MA. Comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkanin. J Nat Prod. 1986; 49:466–469.
Article10. Kim J, Jeong DH, Kim SH, Park SK, Cho Y. Comparative effect of Gromwell (Lithospermum erytheorhizon) extract and Borage oil reversing epidermal proliferation in Guinea Pig. Biosci Biotechnol Biochem. 2006; 70:2086–2095.
Article11. Hayashi M. Pharmacological studies of shikon and tooki. Effect of topical application of the ether extracts and shiunko on inflammatory reactions. Nippon Yakurigaku Zasshi. 1977; 63:205–214.12. Fujita N, Sakaguchi I, Kobayashi H, Ikeda N, Kato Y, Minamin M, et al. An extract of the root of Lithospermum erytheorhizon accelerates wound healing in diabetic mice. Biol Pharm Bull. 2003; 26:329–335.
Article13. Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997; 195:10–19.
Article14. Gary GM, Yardley HJ. Lipid composition of cells isolated from pig, human and rat epidermis. J Lipid Res. 1975; 16:434–440.15. Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000; 41:2071–2082.
Article16. Murgatroyd PR, Coward W. An improved method for estimating changes in whole-body fat and protein mass in man. Br J Nutr. 1989; 62:311–314.
Article17. Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 2003; 89:147–155.
Article18. Arkwright PD, Fujisawa C, Tanaka A, Matsuda H. Mycobacterium vaccae reduces scratching behavior but not the rash in NC mice with eczema: a randomized, blinded, placebo-controlled trial. J Invest Dermatol. 2005; 124:140–143.
Article19. Grassi A, Palermi G, Paradisi M. Study of tolerance and efficacy of cosmetic preparations with lenitive action in atopic dermatitis in children. Clin Ter. 2000; 151:77–80.20. Lodén M. The increase in skin hydration after application of emollients with different amounts of lipids. Acta Derm Venereol. 1992; 72:327–330.21. Tabata N, O'Goshi K, Zhen YX, Kligman AM, Tagami H. Biophysical assessment of persistent effects of moisturizers after their daily applications: evaluation of corneotherapy. Dermatology. 2000; 200:308–313.
Article22. Lodén M, Andersson A-C, Lindberg M. Improvement in skin barrier function in patients with atopic dermatitis after treatment with a moisturizing cream (Canoderm1). Br J Dermatol. 1999; 140:264–267.23. Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991; 24:1–26.
Article24. Wertz PW, Swartzendruber DC, Abraham W, Madison KC, Downing DT. Essential fatty acids and epidermal integrity. Arch Dermatol. 1987; 123:1381–1384.
Article25. Burr GO, Burr MM. A new deficiency disease produced by rigid exclusion of fat from the diet. J Biol Chem. 1929; 82:345–367.
Article26. Chung S, Kong S, Seong K, Cho Y. γ-Linolenic acid in borage oil reverses epidermal hyperproliferation in guinea pigs. J Nutr. 2002; 132:3090–3097.
Article27. Akimoto K, Yoshikawa N, Higaki Y, Kawashima M, Imokawa G. Quantitative analysis of stratum corneum lipids in xerosis and asteatotic eczema. J Dermatol. 1993; 20:1–6.
Article28. Motta S, Monti M, Sesana S, Mellesi L, Ghidoni R, Caputo R. Abnormality of water barrier function in psoriasis. Role of ceramide functions. Arch Dermatol. 1994; 130:452–456.
Article29. Williams Ml. Lipids in normal and pathological desquamation. Adv Lipid Res. 1991; 24:211–262.
Article30. Serup J, Blichmann C. Epidermal hydration of psoriasis plaques and the relation to scaling. Acta Derm Venereol. 1987; 67:357–366.31. Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound ω-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002; 119:166–173.
Article32. Tohyama J, Oya Y, Ezoe T, Vanier MT, Nakayasu H, Fujita N, et al. Ceramide accumulation is associated with increased apoptotic cell death in cultured fibroblasts of sphingolipid activator protein- deficient mouse but not in fibroblasts of patients with Farber disease. J Inher Metab Dis. 1999; 22:649–662.
Article33. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article34. Cho Y, Lew BL, Seong K, Kim NI. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J Korean Med Sci. 2004; 19:859–863.
Article35. Hara J, Hoguchi K, Okamoto R, Kawashima M, Imokawa G. High expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2000; 115:406–413.
Article36. Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998; 78:27–30.
Article37. Melnik B, Hollmann J, Hofmann U, Yuh MS, Plewig G. Lipid composition of outer stratum corneum and nails in atopic and control subjects. Arch Dermatol Res. 1990; 282:549–551.
Article38. Murata Y, Ogata J, Higaki Y, Kawashima M, Yada Y, Higuchi K, et al. Abnormal expression of sphingomyelin acylase in atopic dermatitis: an etiologic factor for ceramide deficiency? J Invest Dermatol. 1996; 106:1242–1249.
Article39. Yamamura T, Tezuka T. Change in sphingomyelinase activity in human epidermis during aging. J Dermatol Sci. 1990; 1:79–83.
Article40. Imokawa G, Hattori M. A possible function of structural lipid in water-holding properties by the stratum corneum. J Invest Dermatol. 1985; 84:282–284.
Article41. Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991; 283:219–223.
Article42. Ballantine JA. The isolation of two esters of the naphthalquinone alcohol, shikonin, from the shrub Jatropha glandulifera. Phytochemistry. 1969; 8:1587–1590.
Article43. Tabata M, Fujita Y. Production of shikonin by plant cell cultures. In : Zaitilin M, Day P, Hollaender A, editors. Biotechnology in Plant Science. San Diego: Academic Press;1985. p. 207–218.44. Kim YS, Kang SH, Jung JH. Studies on the processing of Korean traditional so-ju, Jindo-Hongju. Korean J Dietary Culture. 1991; 6:245–249.45. Kim SJ, Jung JH, Park KH. Studies on the standard method of Jindo Hongju pigments. Korean J Dietary Culture. 1991; 7:19–23.46. Cho MH, Paik YS, Hann TR. Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea. J Agric Food Chem. 1999; 47:4117–4120.
Article47. Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor α promoter in vivo. J Biol Chem. 2004; 279:5877–5885.
Article48. Lu G, Liao J. Detection of the anticancer biological effect of naphtaquinone pigment-LIII. Zhong Xi Yi Jie He Za Zhi. 1990; 10:422–425.49. Yoon Y, Kim YO, Lim NY, Jeon WK, Sung HJ. Shikonin, an ingredient of Lithospermum erythrorhizon-induced apoptosis in HL-60 human premyelocytic leukemia cell line. Planta Med. 1999; 65:532–535.
Article50. Fujii N, Yamashita Y, Arima Y, Nagashima M, Nakano H. Induction of topoisomerase II-mediated DNA cleavage by the plant naphtaquinones plumbagin and shikonin. Antimicrob Agents Chemother. 1992; 36:2589–2594.
Article51. Hashimoto S, Xu M, Masuda Y, Aiuchi T, Nakajo S, Cao J, et al. beta-Hydroxyisovalery lshikonin inhibits the cell growth of various cancer cell lines and induces apoptosis in leukemia HL-60 cells through a mechanism different from those of Fas and etoposide. J Biochem. 1999; 125:17–23.
Article52. Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004; 17:Suppl.1. 43–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the hydration function of cutaneous stratum corneum
- Effect of Delipidization on Binding of Hydrocortisone to Human Stratum Corneum
- Measurement and Comparison of Hydration and Lipid Levels between Patients with Acne vulgaris and Patients with Atopic Dermatitis
- Epidermal Lipid Homeostasis
- Induction of a Hardening Phenomenon and Quantitative Changes of Ceramides in Stratum Corneum