Ann Dermatol.
2016 Apr;28(2):222-231. 10.5021/ad.2016.28.2.222.
The MARCOPOLO Study of Ustekinumab Utilization and Efficacy in a Real-World Setting: Treatment of Patients with Plaque Psoriasis in Asia-Pacific Countries
- Affiliations
-
- 1Department of Dermatology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Dermatology, National Taiwan University Hospital, Taipei, Taiwan.
- 3National Skin Centre, Singapore.
- 4Department of Dermatology, Hospital Sultanah Aminah, Johor Bahru, Malaysia.
- 5Private Practice, Jakarta, Indonesia.
- 6Janssen Asia Pacific, Medical Affairs, Melbourne, Australia.
- 7Janssen China, Biostatistics, Beijing, China.
- 8Department of Dermatology and Cutaneous Biology Research Institute, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. mglee@yuhs.ac
- KMID: 2171381
- DOI: http://doi.org/10.5021/ad.2016.28.2.222
Abstract
- BACKGROUND
Ustekinumab is a fully human monoclonal antibody approved for the treatment of chronic moderate-to-severe plaque psoriasis in adults. However, factors including efficacy, tolerability, ease of use, and cost burden may affect ustekinumab utilization. Noncompliance may, in turn, affect treatment response.
OBJECTIVE
To evaluate ustekinumab utilization in the real-world setting in Asia-Pacific countries.
METHODS
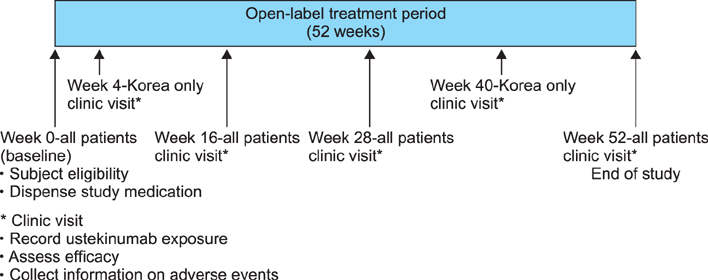
In this phase 4 observational study conducted in Indonesia, Malaysia, Singapore, Korea, and Taiwan, adults with plaque psoriasis receiving ustekinumab were followed for up to 52 weeks. Study endpoints were the proportion of all patients using ustekinumab according to label-recommended intervals and the proportion of Korean patients who achieved a psoriasis area severity index 75 response at week 16. Safety was assessed by monitoring adverse events.
RESULTS
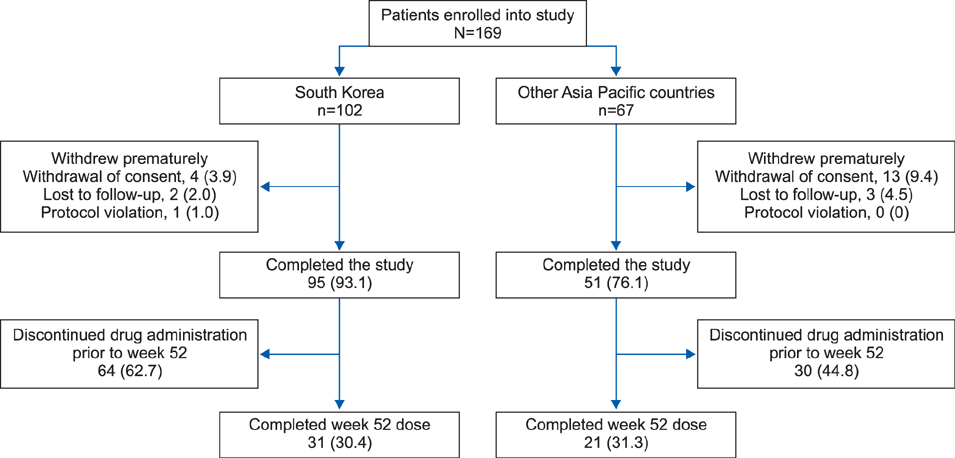
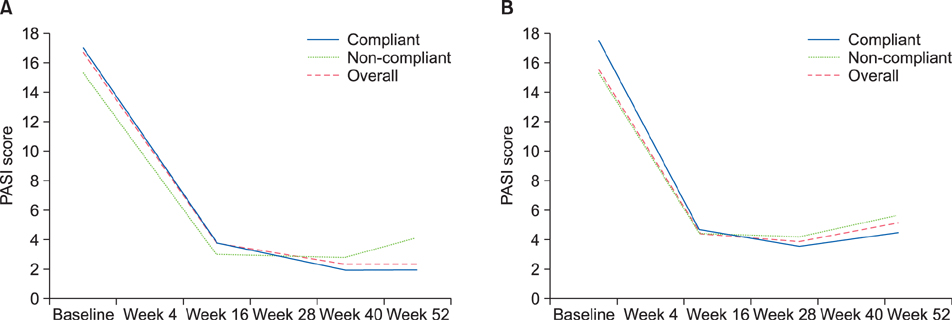
Overall, 169 patients received ustekinumab (Korea, n=102; other countries, n=67). Just over half (56.2%) of patients used ustekinumab with the label-recommended interval from baseline to week 40; the proportion was higher in Korea (73.5%) than in other countries (29.9%), probably because ustekinumab was provided without charge for Korean patients up to week 40. Noncompliance increased after week 40 in Korea and from week 28 in other Asia-Pacific countries, with cost cited as the most common reason. At week 16, 56.9% of Korean patients achieved a Psoriasis Area Severity Index 75 response. Safety results were in line with those seen in previous studies.
CONCLUSION
More than half of all patients in Asia-Pacific countries used ustekinumab as per the label-recommended dose interval, but reimbursement variations between countries may have confounded overall results.
Keyword
MeSH Terms
Figure
Reference
-
1. Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014; 134:1527–1534.
Article2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013; 133:377–385.
Article3. Cingoz O. Ustekinumab. MAbs. 2009; 1:216–221.
Article4. Lee YW, Park EJ, Kwon IH, Kim KH, Kim KJ. Impact of psoriasis on quality of life: relationship between clinical response to therapy and change in health-related quality of life. Ann Dermatol. 2010; 22:389–396.
Article5. Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011; 63:40–46.
Article6. Na SJ, Jo SJ, Youn JI. Clinical study on psoriasis patients for past 30 years (1982-2012) in Seoul National University Hospital Psoriasis Clinic. J Dermatol. 2013; 40:731–735.
Article7. Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010; 34:J314–J321.
Article8. Lee H, Lee MH, Lee DY, Kang HY, Kim KH, Choi GS, et al. Prevalence of vitiligo and associated comorbidities in Korea. Yonsei Med J. 2015; 56:719–725.
Article9. Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol. 2001; 15:16–17.
Article10. O'Neill JL, Kalb RE. Ustekinumab in the therapy of chronic plaque psoriasis. Biologics. 2009; 3:159–168.11. Nestle FO, Conrad C. The IL-12 family member p40 chain as a master switch and novel therapeutic target in psoriasis. J Invest Dermatol. 2004; 123:xiv–xv.
Article12. Lew W, Lee E, Krueger JG. Psoriasis genomics: analysis of proinflammatory (type 1) gene expression in large plaque (Western) and small plaque (Asian) psoriasis vulgaris. Br J Dermatol. 2004; 150:668–676.
Article13. Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007; 80:273–290.
Article14. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008; 371:1665–1674.
Article15. Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008; 371:1675–1684.
Article16. Zhu X, Zheng M, Song M, Shen YK, Chan D, Szapary PO, et al. LOTUS Investigators. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol. 2013; 12:166–174.17. Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. PEARL Investigators. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011; 63:154–163.
Article18. Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012; 39:242–252.
Article19. Reich K, Papp KA, Griffiths CE, Szapary PO, Yeilding N, Wasfi Y, et al. PHOENIX 1, PHOENIX 2, and ACCEPT investigators. An update on the long-term safety experience of ustekinumab: results from the psoriasis clinical development program with up to four years of follow-up. J Drugs Dermatol. 2012; 11:300–312.20. Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013; 27:1535–1545.
Article21. Callis Duffin K, Yeung H, Takeshita J, Krueger GG, Robertson AD, Troxel AB, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014; 170:672–680.
Article22. Yeung H, Wan J, Van Voorhees AS, Callis Duffin K, Krueger GG, Kalb RE, et al. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol. 2013; 68:64–72.
Article23. Stelara® (Ustekinumab). Highlights of prescribing information [Internet]. Horsham, PA: Janssen Biotech, Inc.;2012. updated 2014. cited 2015 Mar. Available at: http://www.stelarainfo.com/pdf/PrescribingInformation.pdf.24. Stelara® (Ustekinumab). Summary of product characteristics [Internet]. Beerse: Janssen-Cilag International NV;2014. updated 2013. cited 2015 Mar. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000958/WC500058513.pdf.25. Youn SW, Choi CW, Kim BR, Chae JB. Reduction of Inter-Rater and Intra-Rater Variability in Psoriasis Area and Severity Index Assessment by Photographic Training. Ann Dermatol. 2015; 27:557–562.
Article26. Youn SW, Kim BR, Lee JH, Song HJ, Choe YB, Choi JH, et al. Comparison of treatment goals for moderate-to-severe psoriasis between Korean dermatologists and the European consensus report. Ann Dermatol. 2015; 27:184–189.
Article27. Chiu HY, Chen CH, Wu MS, Cheng YP, Tsai TF. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013; 169:1295–1303.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Economic Factors as Major Determinants of Ustekinumab Drug Survival of Patients with Chronic Plaque Psoriasis in Korea
- A Case of Paradoxical Flare of Pustular Psoriasis after Ustekinumab Therapy
- Safety of Adalimumab, Ustekinumab, Secukinumab, Guselkumab in Korean Patients with Moderate to Severe Psoriasis: A Real-World Data in a Single Medical Center
- Toenail Psoriasis during Ustekinumab Therapy: Results and Limitations
- Efficacy and Safety of Ustekinumab in the Treatment of Moderate to Severe Psoriasis in Korea