Infect Chemother.
2012 Jun;44(3):175-179. 10.3947/ic.2012.44.3.175.
Effect of Ritonavir-boosting on Atazanavir Discontinuation due to Jaundice in HIV-infected Koreans
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. wbpark1@snu.ac.kr
- KMID: 2170367
- DOI: http://doi.org/10.3947/ic.2012.44.3.175
Abstract
- BACKGROUND
Data regarding differences of intolerance between a ritonavir-unboosted and a ritonavir-boosted atazanavir regimen in HIV-infected Koreans is limited.
MATERIALS AND METHODS
A review was conducted of the incidence of severe hyperbilirubinemia (serum total bilirubin >3.1 mg/dL) and discontinuation of atazanavir in HIV-infected patients who had received an atazanavir-containing regimen at Seoul National University Hospital from 2005 to 2009. Patients with active liver disease were excluded from the study.
RESULTS
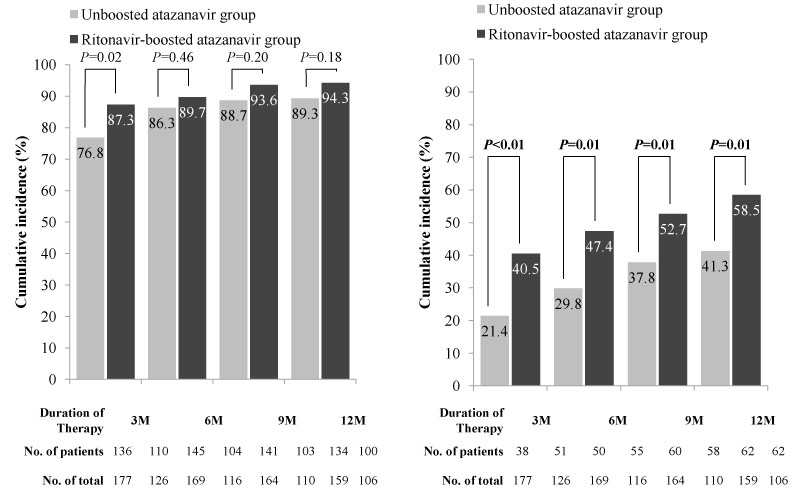
Of a total of 335 patients receiving an atazanavir-containing regimen, 145 (43.3%) received treatment with a ritonavir-boosted regimen. The cumulative incidence of severe hyperbilirubinemia at three months was 40.5% in patients receiving a ritonavir-boosted atazanavir regimen and 21.4% in patients receiving an un-boosted atazanavir regimen (P<0.001). The cumulative incidence of severe hyperbilirubinemia at 12 months was 58.5% in patients receiving a ritonavir-boosted regimen and 41.3% in those receiving an un-boosted regimen (P=0.008). The proportion of drug discontinuation due to jaundice during the 12-month period was 11.7% in patients receiving a ritonavir-boosted regimen and 5.3% in those receiving an un-boosted regimen (P=0.035).
CONCLUSIONS
Occurrence of severe hyperbilirubinemia and discontinuation of atazanavir due to jaundice was significantly more common in HIV-infected Koreans who received a ritonavir-boosted atazanavir regimen than in those who received a ritonavir-un-boosted atazanavir regimen.
Keyword
MeSH Terms
Figure
Reference
-
1. Havlir DV, O'Marro SD. Atazanavir: new option for treatment of HIV infection. Clin Infect Dis. 2004. 38:1599–1604.
Article2. Zucker SD, Qin X, Rouster SD, Yu F, Green RM, Keshavan P, Feinberg J, Sherman KE. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci USA. 2001. 98:12671–12676.
Article3. Squires K, Lazzarin A, Gatell JM, Powderly WG, Pokrovskiy V, Delfraissy JF, Jemsek J, Rivero A, Rozenbaum W, Schrader S, Sension M, Vibhagool A, Thiry A, Giordano M. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004. 36:1011–1019.
Article4. Wood R, Phanuphak P, Cahn P, Pokrovskiy V, Rozenbaum W, Pantaleo G, Sension M, Murphy R, Mancini M, Kelleher T, Giordano M. Long-term efficacy and safety of atazanavir with stavudine and lamivudine in patients previously treated with nelfinavir or atazanavir. J Acquir Immune Defic Syndr. 2004. 36:684–692.
Article5. Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, Lichtenstein K, Rightmire A, Sankoh S, Wilber R. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005. 19:685–694.
Article6. Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, Lichtenstein K, Wirtz V, Rightmire A, Odeshoo L, McLaren C. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006. 20:711–718.
Article7. Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother. 2004. 53:4–9.
Article8. Malan DR, Krantz E, David N, Wirtz V, Hammond J, McGrath D. Efficacy and safety of atazanavir, with or without ritonavir, as part of once-daily highly active antiretroviral therapy regimens in antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2008. 47:161–167.
Article9. Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, Moyle G, Mancini M, Percival L, Yang R, Thiry A, McGrath D. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008. 372:646–655.
Article10. Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, Moyle G, Mancini M, Percival L, Yang R, Wirtz V, Lataillade M, Absalon J, McGrath D. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010. 53:323–332.
Article11. The Korean Society for AIDS. Clinical Guidelines for the Diagnosis and Treatment of HIV/AIDS in HIV-infected Koreans. Infect Chemother. 2011. 43:89–128.12. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011. 10. 14. Department of Health and Human Services;1–167. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.13. Ki CS, Lee KA, Lee SY, Kim HJ, Cho SS, Park JH, Cho S, Sohn KM, Kim JW. Haplotype structure of the UDP-glucuronosyltransferase 1A1 (UGT1A1) gene and its relationship to serum total bilirubin concentration in a male Korean population. Clin Chem. 2003. 49:2078–2081.
Article14. Choe PG, Park WB, Song JS, Kim NH, Song KH, Park SW, Kim HB, Kim NJ, Oh MD. Incidence of atazanavir-associated hyperbilirubinemia in Korean HIV patients: 30 months follow-up results in a population with low UDP-glucuronosyltransferase 1A1*28 allele frequency. J Korean Med Sci. 2010. 25:1427–1430.
Article15. Park WB, Choe PG, Song KH, Jeon JH, Park SW, Kim HB, Kim NJ, Oh MD, Choe KW. Genetic factors influencing severe atazanavir-associated hyperbilirubinemia in a population with low UDP-glucuronosyltransferase 1A1*28 allele frequency. Clin Infect Dis. 2010. 51:101–106.
Article16. Fellay J, Boubaker K, Ledergerber B, Bernasconi E, Furrer H, Battegay M, Hirschel B, Vernazza P, Francioli P, Greub G, Flepp M, Telenti A. Prevalence of adverse events associated with potent antiretroviral treatment: Swiss HIV Cohort Study. Lancet. 2001. 358:1322–1327.
Article17. Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995. 333:1171–1175.
Article18. Rotger M, Taffe P, Bleiber G, Gunthard HF, Furrer H, Vernazza P, Drechsler H, Bernasconi E, Rickenbach M, Telenti A. Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. J Infect Dis. 2005. 192:1381–1386.
Article19. Ray JE, Marriott D, Bloch MT, McLachlan AJ. Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. Br J Clin Pharmacol. 2005. 60:291–299.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Multidrug-Resistant Human Immunodeficiency Virus Infection Treated with Atazanavir and Lopinavir/Ritonavir Combination Therapy
- Incidence of Atazanavir-associated Hyperbilirubinemia in Korean HIV Patients: 30 Months Follow-up Results in a Population with Low UDP-glucuronosyltransferase1A1*28 Allele Frequency
- Clinical Guidelines for the Treatment and Prevention of Opportunistic Infections in HIV-infected Koreans
- Lipid Profile Changes after Switch to Atazanavir from other Protease Inhibitor-based Combined Antiretroviral Treatment in HIV-infected Korean
- Alopecia Areata Associated with Abacavir Therapy