J Bacteriol Virol.
2015 Dec;45(4):339-353. 10.4167/jbv.2015.45.4.339.
The Preparedness for Re-emerged and Emerging Infectious Diseases: A Lesson Through Outbreak of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in South Korea
- Affiliations
-
- 1Department of Microbiology and Immunology, and Global Center for Infectious Diseases, Seoul National University College of Medicine, Seoul, Korea. hesss@snu.ac.kr, lamseok@snu.ac.kr
- 2Institute of Endemic Disease, Seoul National University Medical Research Center, Seoul, Korea.
- KMID: 2168718
- DOI: http://doi.org/10.4167/jbv.2015.45.4.339
Abstract
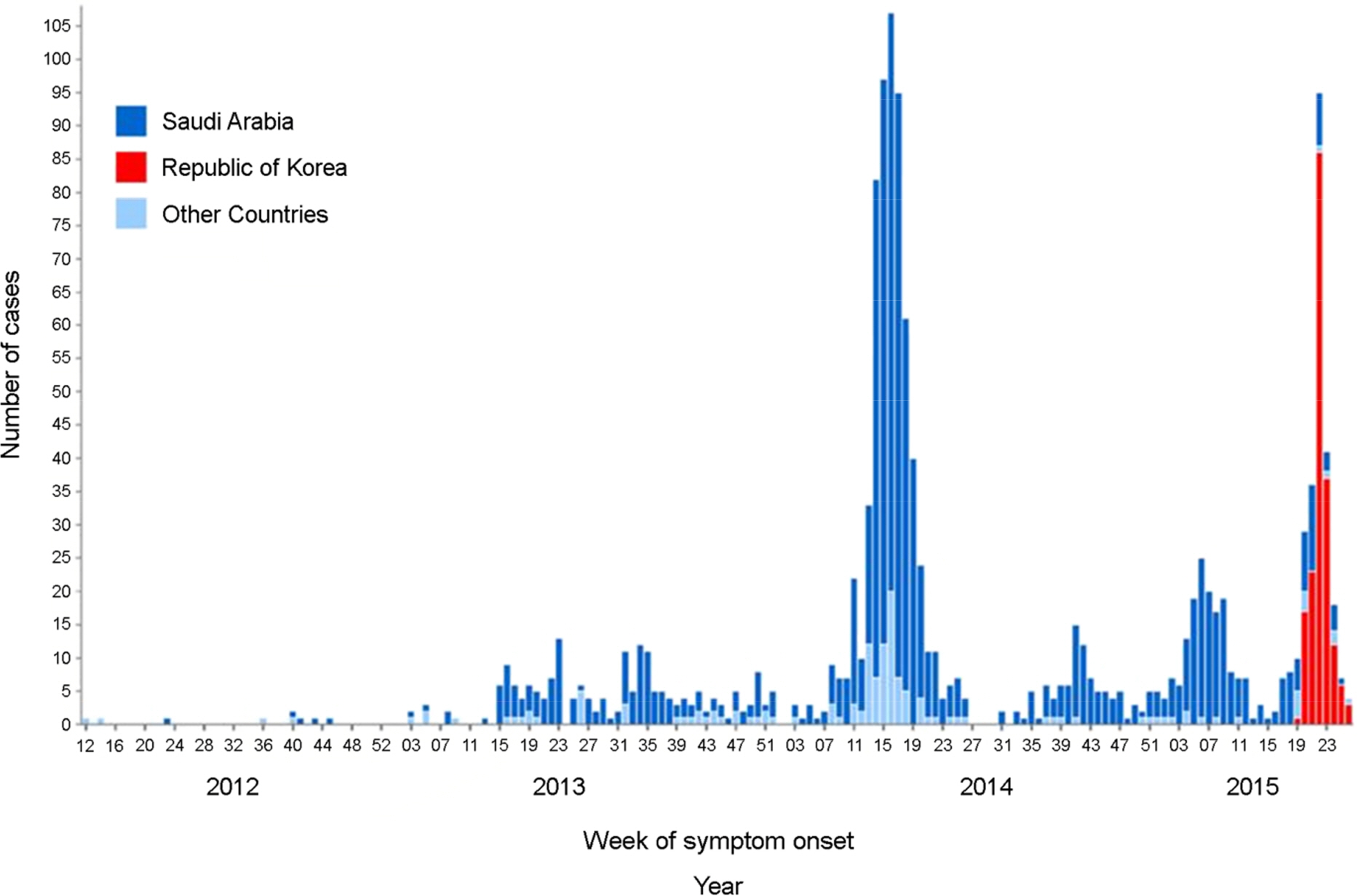
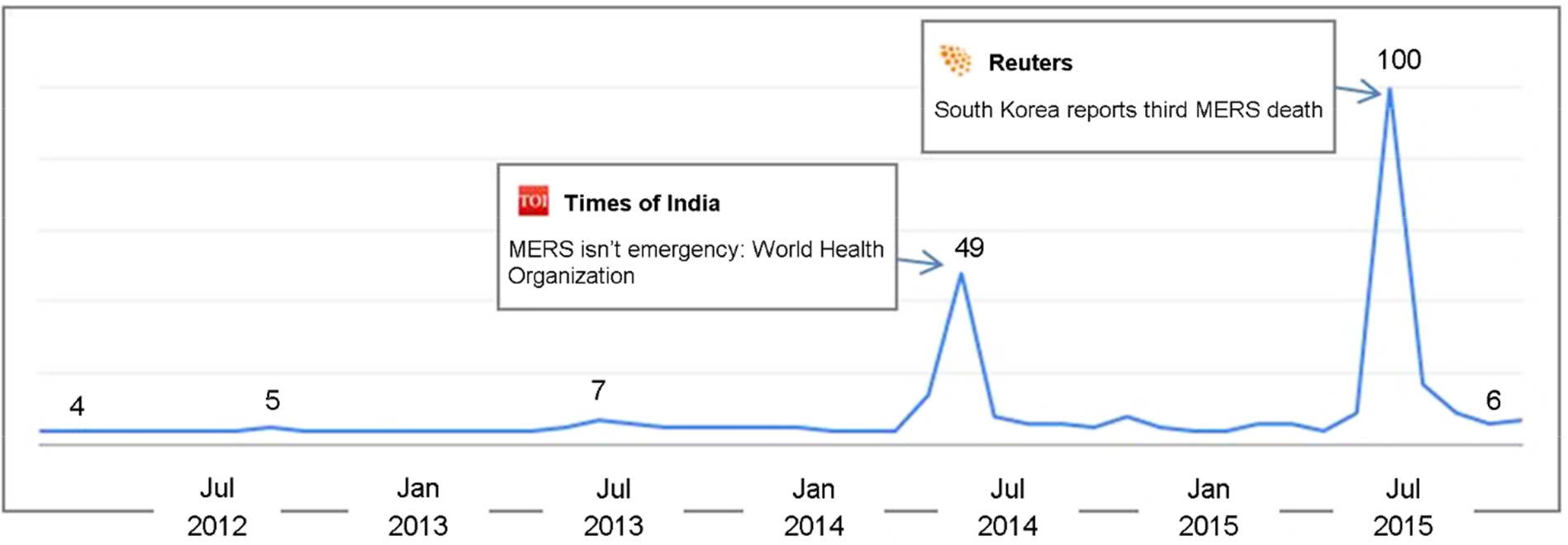
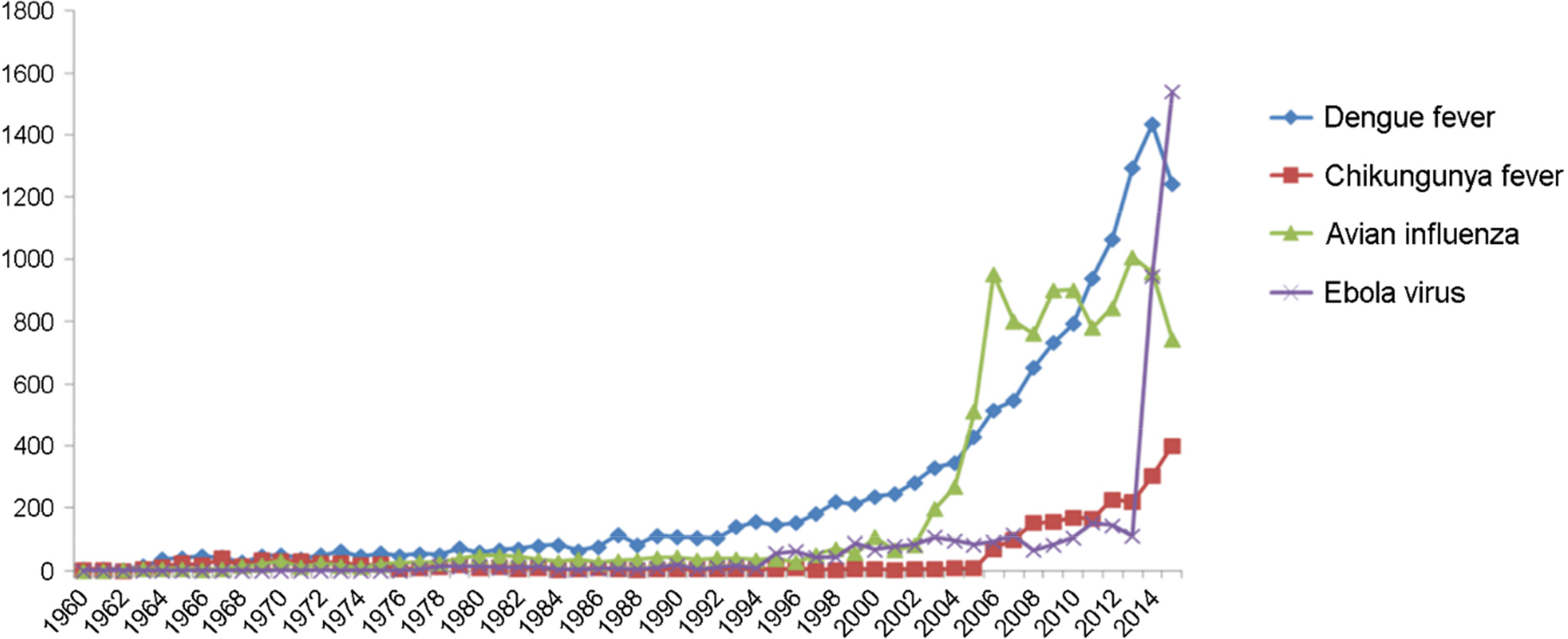
- The Middle East respiratory syndrome coronavirus (MERS-CoV) causes severe acute respiratory disease and systemic dysfunction that may eventually lead to the death of the patients. After MERS-CoV was first diagnosed in the South Korea, in May 2015, it affected 186 individuals and claimed 37 lives in short span of time (case fatality rate = 19.9%). Compared to MERS-CoV in the Middle East, MERS-CoV in South Korea appeared to be more transmissible, and induced multiple human-to-human transmission. These knowledge gaps caused the failure of early prevention, and disseminated MERS-CoV brought out a great loss of lives and economy. The MERS-CoV outbreak revealed the potential weakness of public health system in South Korea, and promoted the reestablishment of preventive strategies for imported infectious diseases. In these regards, we analyzed the potential for additional import of re-emerged and emerging infectious diseases, such as dengue fever, malaria, chikungunya fever and hepatitis A, from Africa or South-East Asia. Then we suggest the investment expansion and the administration of global networks for effective research and control for newly or re-emerged infectious diseases. In conclusion, it is required to expect and prepare for the surveillance of the importation of foreign pathogens, and constitute the internal collaborative systems for rapid detection and risk communication. In addition, we should take an active part in the global networks to perform rapid preparedness and control for re-emerged or emerging infectious diseases.
Keyword
MeSH Terms
Figure
Reference
-
1). Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012; 367:1814–20.
Article2). Al-Abdallat MM, Payne DC, Alqasrawi S, Rha B, Tohme RA, Abedi GR, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014; 59:1225–33.
Article3). World Health Organization. Summary of Current Situation. Literature Update and Risk Assessment. July 2015. http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-7july2015/en/. (Accessed 1 Oct, 2015).4). Ithete NL, Stoffberg S, Corman VM, Cottontail VM, Richards LR, Schoeman MC, et al. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013; 19:1697–9.
Article5). Lau SK, Li KS, Tsang AK, Lam CS, Ahmed S, Chen H, et al. Genetic characterization of Betacoronavirus lineage C viruses in bats reveals marked sequence divergence in the spike protein of pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications for the origin of the novel Middle East respiratory syndrome coronavirus. J Virol. 2013; 87:8638–50.
Article6). Kayali G, Peiris M. A more detailed picture of the epidemiology of Middle East respiratory syndrome coronavirus. Lancet Infect Dis. 2015; 15:495–7.
Article7). Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus: transmission and phylogenetic evolution. Trends Microbiol. 2014; 22:573–9.
Article8). Xie Q, Cao Y, Su J, Wu X, Wan C, Ke C, et al. Genomic sequencing and analysis of the first imported Middle East Respiratory Syndrome Coronavirus (MERS CoV) in China. Sci China Life Sci. 2015; 58:818–20.
Article9). World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV): Epicurve of confirmed cases and deaths in Republic of Korea, China, Saudi Arabia and other countries. July 2015. http://www.who.int/emergencies/mers-cov/en/. (Accessed 1 Oct, 2015).10). Korea Centers for Disease Control and Prevention. Middle East Respiratory Syndrome Information. June 2015. http://www.mers.go.kr/mers/html/jsp/main.jsp. (Accessed 1 Oct, 2015).11). Reusken CB, Ababneh M, Raj VS, Meyer B, Eljarah A, Abutarbush S, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill. 2013; 18:20662.
Article12). Reusken CB, Haagmans BL, Müller MA, Gutierrez C, Godeke GJ, Meyer B, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013; 13:859–66.
Article13). Hemida MG, Perera RA, Wang P, Alhammadi MA, Siu LY, Li M, et al. Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013; 18:20659.
Article14). Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014; 14:140–5.
Article15). Chu DK, Poon LL, Gomaa MM, Shehata MM, Perera RA, Abu Zeid D, et al. MERS coronaviruses in dromedary camels, Egypt. Emerg Infect Dis. 2014; 20:1049–53.
Article16). Reusken CB, Messadi L, Feyisa A, Ularamu H, Godeke GJ, Danmarwa A, et al. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis. 2014; 20:1370–4.
Article17). Drosten C, Meyer B, Müüller MA, Corman VM, Al-Masri M, Hossain R, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014; 371:828–35.
Article18). Suwantarat N, Apisarnthanarak A. Risks to healthcare workers with emerging diseases: lessons from MERS-CoV, Ebola, SARS, and avian flu. Curr Opin Infect Dis. 2015; 28:349–61.19). Müller MA, Meyer B, Corman VM, Al-Masri M, Turkestani A, Ritz D, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015; 15:559–64.
Article20). Du L, Jiang S. Middle East respiratory syndrome: current status and future prospects for vaccine development. Expert Opin Biol Ther. 2015; 15:1647–51.
Article21). Guo X, Deng Y, Chen H, Lan J, Wang W, Zou X, et al. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology. 2015; 145:476–84.
Article22). Volz A, Kupke A, Song F, Jany S, Fux R, Shams-Eldin H, et al. Protective efficacy of recombinant Modified Vaccinia virus Ankara (MVA) delivering Middle East Respiratory Syndrome coronavirus spike glycoprotein. J Virol. 2015; 89:8651–6.23). Mou H, Raj VS, van Kuppeveld FJ, Rottier PJ, Haagmans BL, Bosch BJ. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013; 87:9379–83.
Article24). Du L, Zhao G, Kou Z, Ma C, Sun S, Poon VK, et al. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol. 2013; 87:9939–42.
Article25). Muthumani K, Falzarano D, Reuschel EL, Tingey C, Flingai S, Villarreal DO, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015; 7:301ra132.
Article26). Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014; 32:3169–74.
Article27). National geographic. Why a MERS Vaccine Won't Be Easy. May 2014. http://news.nationalgeographic.com/news/2014/05/140523-mers-vaccine-camels-virus-flu-saudi-pandemic-science/. (Accessed Oct 1, 2015).28). Butler D. South Korean MERS outbreak spotlights lack of research. Nature. 2015; 522:139–40.
Article29). Banik GR, Khandaker G, Rashid H. Middle East Respiratory Syndrome Coronavirus "MERS-CoV": Current Knowledge Gaps. Paediatr respir rev. 2015; 16:197–202.
Article30). Ministry of Government Legislation. Disease Prevention and Control Law of 2015. Law No. 13392. Act of 6 July 2015.31). Korea Centers for Disease Control and Prevention. Full disclosure of MERS-hit hospitals. June 2015. http://www.cdc.go.kr/CDC/intro/CdcKrIntro0201.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0011&cid=63293. (Accessed 1 Oct, 2015).32). Korea Centers for Disease Control and Prevention. National Reorganization of Disease Control System. Sep. 2015. http://www.cdc.go.kr/CDC/intro/CdcKrIntro0201.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0011&cid=65273. (Accessed 1 Oct, 2015).33). Korea Centers for Disease Control and Prevention. Response Guidelines for MERS. May 2015. http://www.cdc.go.kr/CDC/info/CdcKrHealth0289.jsp?menuIds=HOME001-MNU1132-MNU1013-MNU1913&cid=63072. (Accessed 1 Oct, 2015).34). Korea Centers for Disease Control and Prevention. Middle East Respiratory Syndrome Information. June 2015. http://www.mers.go.kr/mers/html/jsp/Menu_B/content_B1.jsp?cid=26740. (Accessed 1 Oct, 2015).35). Korean Hospital Association. Compensation for Loss of Hospital in MERS Outbreak. Sep. 2015. https://www.kha.or.kr/impart/notice/view. (Accessed 1 Oct, 2015).36). National Institutes of Health, Study of Ebola survivors opens in Liberia. June 2015. http://www.nih.gov/news/health/jun2015/niaid-17.htm. (Accessed 1 Oct, 2015).37). Korea Tourism Organization. Statistics of Korean Tourism. July 2015. http://kto.visitkorea.or.kr/kor/notice/data/statis/profit/board/view.kto?id=379196&instanceId=294. (Accessed 1 Oct, 2015).38). Korea Tourism Organization. Statistics of Korean Departures. Aug. 2015. http://kto.visitkorea.or.kr/kor/notice/data/statis/profit/board/view.kto?id=425044&isNotice=false&instanceId=294&rnum=6. (Accessed 1 Oct, 2015).39). Korea Centers for Disease Control and Prevention. Infectious disease annual report of 2014. July 2014. http://www.cdc.go.kr/CDC/info/CdcKrInfo0302.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0038&fid=32&q_type=&q_value=&cid=63970&pageNum=. (Accessed 1 Oct, 2015).40). Korea Centers for Disease Control and Prevention. Travelers Health Information. Oct. 2015. http://travelinfo.cdc.go.kr/travelinfo/jsp_travelinfo/infect_info/contents_gita/disease2_01_1.jsp. (Accessed 1 Oct, 2015).41). Pubmed. Results by year. http://www.ncbi.nlm.nih.gov/pubmed/. (Accessed 15 Oct, 2015).42). Ministry of Strategy and Finance. 2016 Budget. Sep. 2015. http://budget.go.kr/info/2016/budget2016_overview.html. (Accessed 1 Oct, 2015).43). Zhou Y, Sullivan NJ. Immunology and evolvement of the adenovirus prime, MVA boost Ebola virus vaccine. Curr Opin Immunol. 2015; 35:131–6.
Article44). Nakayama Y, Aruga A. Comparison of Current Regulatory Status for Gene-Based Vaccines in the US, Europe and Japan. Vaccines. 2015; 3:186–202.
Article45). Fusco ML, Hashiguchi T, Cassan R, Biggins JE, Murin CD, Warfield KL, et al. Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs. PLoS Pathog. 2015; 11:e1005016.
Article46). Heymann DL, Chen L, Takemi K, Fidler DP, Tappero JW, Thomas MJ, et al. Global health security: the wider lessons from the west African Ebola virus disease epidemic. Lancet. 2015; 385:1884–901.
Article47). Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS). July 2015. http://wwwn.cdc.gov/nndss/nedss.html. (Accessed 1 Oct, 2015).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Same Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) yet Different Outbreak Patterns and Public Health Impacts on the Far East Expert Opinion from the Rapid Response Team of the Republic of Korea
- Institutional Preparedness to Prevent Future Middle East Respiratory Syndrome Coronavirus-Like Outbreaks in Republic of Korea
- Lessons Learned from SARS-CoV and MERS-CoV: Preparation for SARS-CoV-2 induced COVID-19
- Middle East respiratory syndrome: what we learned from the 2015 outbreak in the Republic of Korea
- Collaborative Intervention of Middle East Respiratory Syndrome: Rapid Response Team